Senescence Resarch and Mitochondria
Cellular Senescence and Mitochondria at a Glance
-
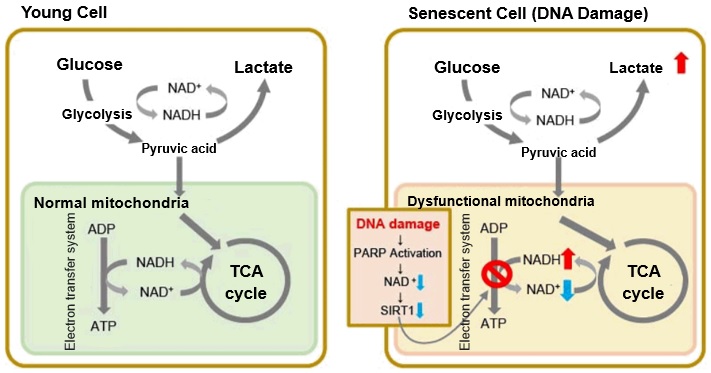
In aged cells, due to mitochondrial dysfunction, ATP is primarily generated through the anaerobic glycolysis pathway, leading to an increase in lactate production2). DNA damage is one of the causes of mitochondrial dysfunction in cellular aging. The accumulation of DNA damage activates DNA repair mechanisms and increases NAD+ consumption. The decrease in NAD+ levels reduces SIRT1 activity, an important factor in maintaining mitochondrial function, leading to impaired mitochondrial function (inhibition of electron transfer → ATP production / reduction of NAD+ levels)1),3).
Reference:
1. J. Wu, Z. Jin, H. Zheng and L. Yan, “Sources and implications of NADH/NAD+redox imbalance in diabetes and its complications”, Diabetes Metab. Syndr. Obes., 2016, 9, 145
2. Z. Feng, R. W. Hanson, N. A. Berger and A. Trubitsyn, “Reprogramming of energy metabolism as a driver of aging”, Oncotarget., 2016, 7(13), 15410.
3. S. Imai and L. Guarente, “NAD+ and sirtuins in aging and disease”, Trends in Cell Biology, 2014, 24(8), 464.
-
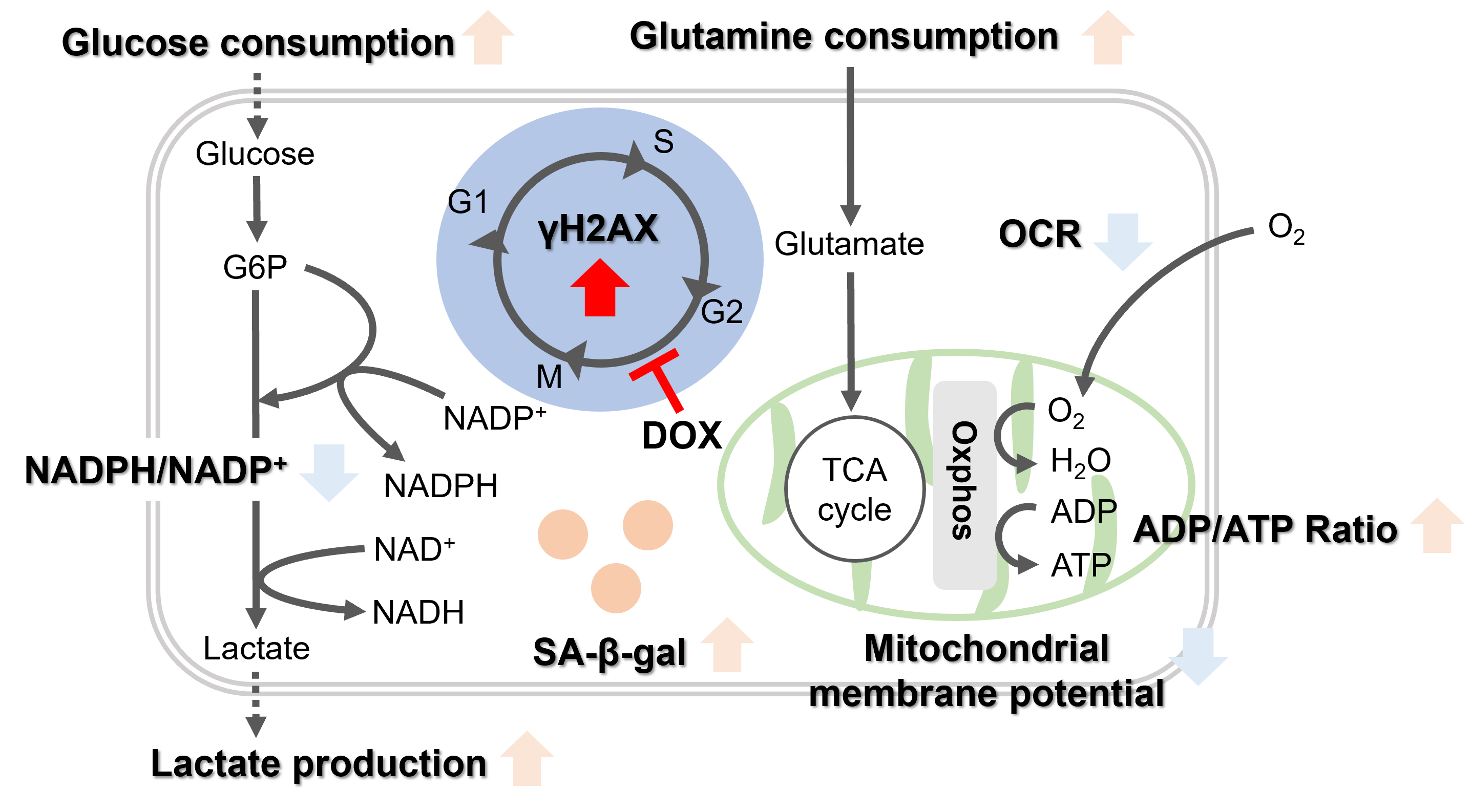
Oxidative stress & accelerated aging: ①SA-β-gal Impairment of mitochondrial function: ③ Mitochondrial membrane potential ④ Oxygen consumption rate (OCR)
-
Upregulation of glycolysis pathway and glutamine metabolism ⑥ Glucose consumption ⑦ Lactate production ⑧ Glutamine consumption
-
Reduction in antioxidant capacity: ⑨ NADPH/NADP+ ratio
-
DNA repair mechanisms:
Key Literature on Mitophagy and Aging
| Title | Neuronal induction of BNIP3-mediated mitophagy slows systemic aging in Drosophila Schmid, E. et al., Nature Aging, 2022, 2, 494-507 |
| Key Points |
1. Aging leads to a decline in mitophagy in the brain of Drosophila, while mitochondrial mass increases. 2. Inducing BNIP3 in the adult nervous system induces mitophagy and prevents the accumulation of dysfunctional mitochondria in the aging brain. 3. Neuronal induction of BNIP3-mediated mitophagy extends the organism's lifespan and healthspan. 4. BNIP3-mediated mitophagy in the nervous system improves muscle and gut homeostasis in aging flies. |
| Title |
Mitochondrial contribution to lipofuscin formation |
| Key Points |
1. Impaired mitophagy in aged cells leads to increased mitochondrial mass and superoxide formation. Additionally, inhibiting mitochondrial fission also increases lipofuscin formation. 2. Downregulation of Lon protease is associated with increased lipofuscin formation, while the application of mitochondrial-targeted antioxidant mitoTEMPO prevents the accumulation of these protein aggregates. |
| Title | Age-associated changes in human CD4+ T cells point to mitochondrial dysfunction consequent to impaired autophagy Bektas, A. et al., Aging, 2019, 11(21), 9234-9263 |
| Key Points |
1. Aging is associated with persistent mitochondrial dysfunction in CD4+ T lymphocytes, and defects in mitophagy turnover may trigger chronic inflammation and lead to impaired immune defense in the elderly. 2. These references and insights highlight the connection between mitochondrial dysfunction, impaired mitophagy, and aging, supporting the critical role of mitochondrial quality control in cellular aging and age-related diseases. |
Accumulation of Lipid Peroxides and Their Connection to Cellular Senescence and Mitochondria
Lipotoxicity is caused by intracellular lipid accumulation and is indicative of mitochondrial disfunction. Lipotoxicity accelerates the degenerative process of cellular senescence, influencing cancer development.
References
1. Clara, C. al., “Mitochondria: Are they causal players in cellular senescence?”, Biochimica et Biophysica Acta – Bioenergetics, 2015, 1847(11), 1373-1379.
2. Huizhen, Z. et al., “Lipidomics reveals carnitine palmitoyltransferase 1C protects cancer cells from lipotoxicity and senescence”, Journal of Pharmaceutical Analysis, 2020.
3. Xiaojuan, H. et al., “Astrocyte Senescence and Alzheimer’s Disease: A Review”, Front. Aging Neurosci., 2020.
4. Borén, J. et al., “Apoptosis-induced mitochondrial dysfunction causes cytoplasmic lipid droplet formation”, Cell Death Differ, 2012, 19(9), 1561-1570.
5. Na, L. et al., “Aging and stress induced β cell senescence and its implication in diabetes development”, Aging (Albany NY), 2019, 11(21), 9947–9959.
Mitochondrial and Lysosomal, and Iron Regulation of Senescence
|
Senescence is a cellular process that results in the cessation of cell division, often serving as a protective mechanism against the proliferation of damaged cells, including potential cancer cells. This process is intricately regulated by numerous factors including, but not limited to, tumor suppressor genes, DNA damage response (DDR) pathways, and various signaling molecules. In addition, the senescence-associated secretory phenotype (SASP), consisting of cytokines, growth factors, and proteases, is regulated by NF-κB and other transcription factors that influence the tissue microenvironment and impact aging and disease processes. |
|
| Related Technique in This Topic |
|
|
|
|
|
|
|
Related Applications |
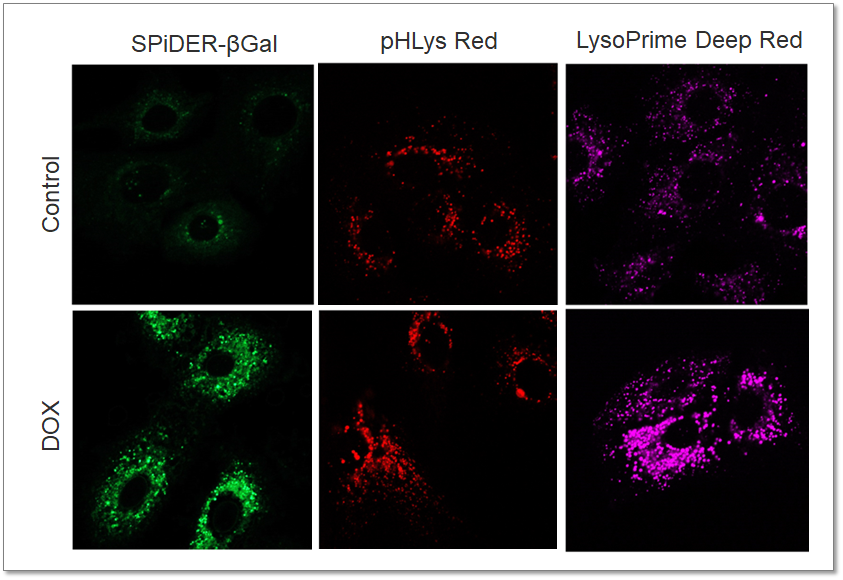
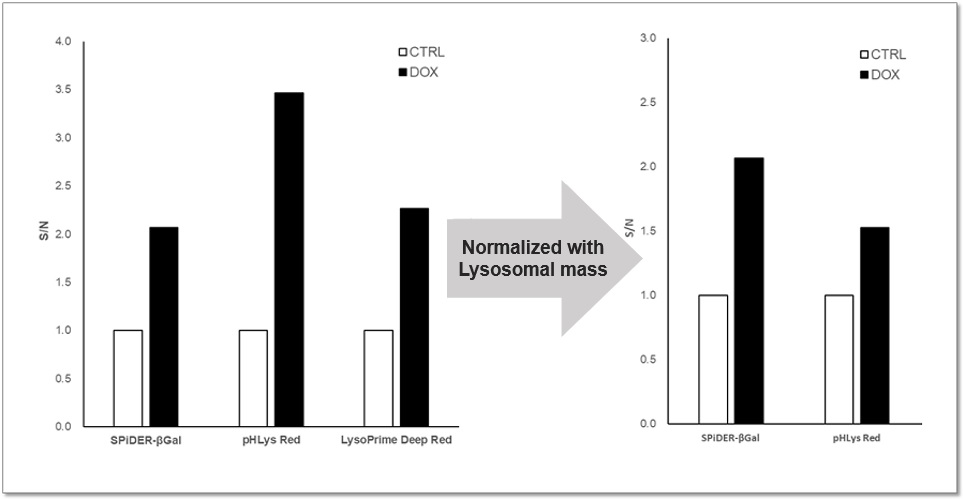
Analysis of Lysosomal Mass and pH change in Senescence-induced Cells
|