Recent research shows that mitochondria are critical for T cell function, regulating metabolic pathways essential for immune responses. Here are some of the papers that show mechanisms and strategies, such as mitochondrial transfer or genetic modification, to improve mitochondrial health and reinvigorate T cell activity against tumours.
Mitochondria play a critical role in T cell function, providing energy and regulating metabolic pathways essential for immune responses. In cancer, the tumour microenvironment induces mitochondrial dysfunction in T cells, leading to exhaustion and reduced anti-tumour efficacy. Strategies to improve mitochondrial health, such as mitochondrial transfer or genetic modification, have shown promise in reinvigorating T cell activity against tumours. Targeting mitochondrial pathways in T cells offers a novel approach to improving cancer immunotherapies and overcoming tumour-induced immunosuppression.
|
-
Intercellular nanotube-mediated mitochondrial transfer enhances T cell metabolic fitness and antitumor efficacy
Click here for the original article: Jeremy G. Baldwin, et. al., Cell, 2024.
Point of Interest
- Bone marrow stromal cells transfer mitochondria to CD8+ T cells via nanotubes, requiring Talin 2 for optimal transfer.
- Mitochondria-boosted T cells exhibit improved respiration, tumor infiltration, and reduced exhaustion, enhancing antitumor responses.
- Intercellular mitochondrial transfer offers a new organelle-based therapy, advancing next-generation cell therapies for cancer treatment.
-
PGE2 inhibits TIL expansion by disrupting IL-2 signalling and mitochondrial function
Click here for the original article: Matteo Morotti, et. al., Nature, 2024.
Point of Interest
- Prostaglandin E2 (PGE2) impairs IL-2 sensing in CD8+ tumour-infiltrating lymphocytes (TILs) by downregulating IL-2 receptor components, causing oxidative stress and cell death.
- Blocking PGE2 signalling during TIL expansion restores IL-2 responsiveness, increasing TIL proliferation and improving tumour control in adoptive cell therapy.
- Targeting PGE2 pathways enhances IL-2-driven T cell expansion, offering new strategies to improve cancer immunotherapy outcomes.
-
FOXO1 enhances CAR T cell stemness, metabolic fitness and efficacy
Click here for the original article: Jack D. Chan, et. al., Nature, 2024.
Point of Interest
- CAR T-cell therapy is less effective in solid tumours due to the immunosuppressive microenvironment that causes T-cell dysfunction.
- Overexpression of FOXO1 enhances the stem-like phenotype, mitochondrial fitness and persistence of CAR T cells, thereby improving antitumour efficacy.
- Engineering FOXO1 in CAR T cells offers a promising strategy to increase efficacy against solid tumours in cancer therapy.
|
| Related Techniques |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Related Applications |
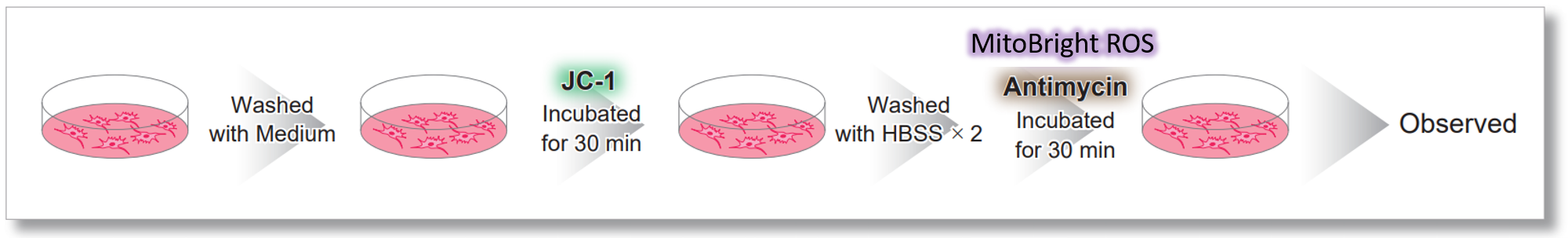
Simultaneously evaluation of mitochondrial superoxide and membrane potential
After HeLa cells were washed with HBSS, co-stained with MitoBright ROS Deep Red and mitochondrial membrane potential staining dye (JC-1: code MT09), and the generated mitochondrial ROS and membrane potential were observed simultaneously. As a result, the decrease in mitochondrial membrane potential and the generation of mitochondrial ROS are simultaneously observed.
-
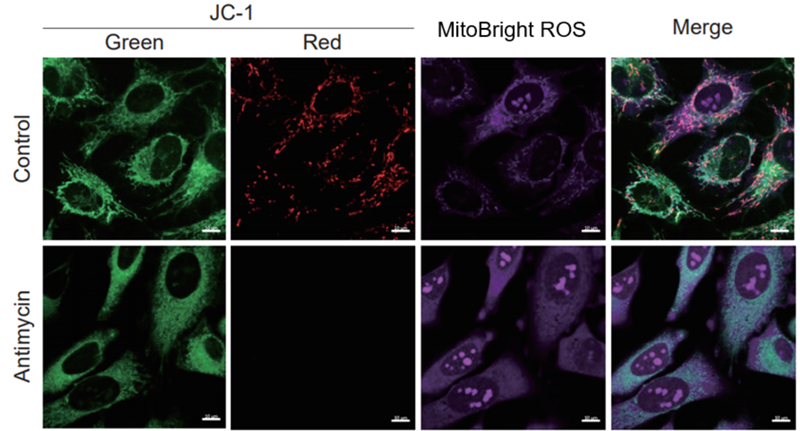
-
<Imaging Conditions>(Confocal microscopy)
JC-1: Green Ex = 488, Em = 490-520 nm, Red: Ex = 561, Em = 560-600 nm
MitoBright ROS :Ex = 633 nm, Em = 640-700 nm
Scale bar: 10 μm
-
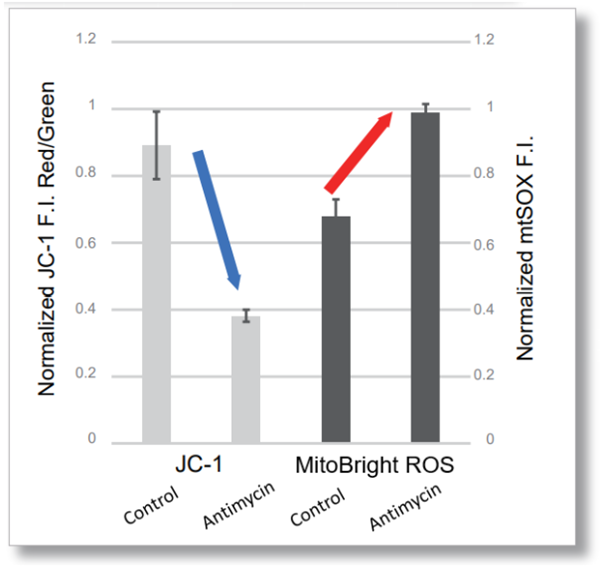
-
<Examination Conditions>(Plate Reader)Tecan, Infinite M200 Pro
JC-1: Green Ex=480-490 nm, Em=525-545 nm; Red: Ex= 530-540 nm, Em=585-605 nm
MitoBright ROS: Ex=545-555 nm, Em = 665-685 nm
|
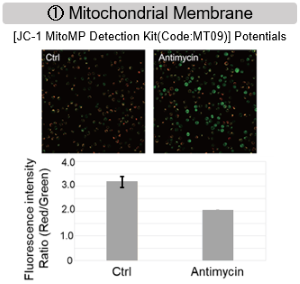
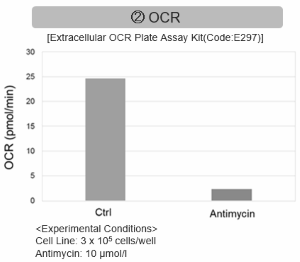
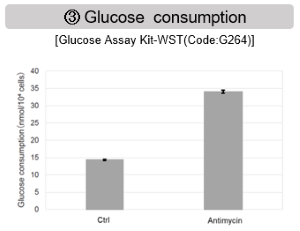
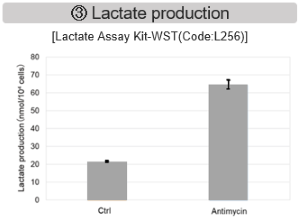
Inhibition of Mitochondrial Electron Transport Chain
-
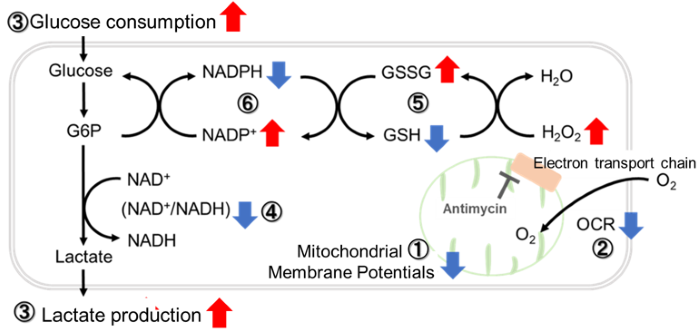
-
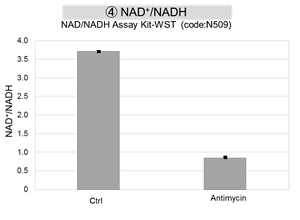
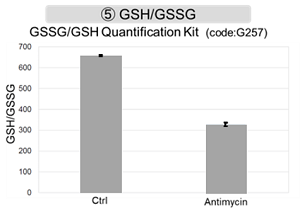
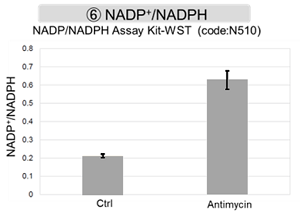
Antimycin stimulation of Jurkat cells was used to evaluate the changes in cellular state upon inhibition of the mitochondrial electron transport chain using a variety of indicators.
The results showed that inhibition of the electron transport chain resulted in (1) a decrease in mitochondrial membrane potential and (2) a decrease in OCR. In addition, (3) the NAD+/NADH ratio of the entire glycolytic pathway decreased due to increased metabolism of pyruvate to lactate to maintain the glycolytic pathway, (4) GSH depletion due to increased reactive oxygen species (ROS), and (6) increase in the NADP+/NADPH ratio due to decreased NADH required for glutathione biosynthesis were observed.
|