ROS Assay Kit -Photo-oxidation Resistant DCFH-DA-
ROS Detection
-
Product codeR253 ROS Assay Kit -Photo-oxidation Resistant DCFH-DA-
| Unit size | Price | Item Code |
|---|---|---|
| 100 tests | $324.00 | R253-10 |

Refer to experimental example: live cell high content imaging of ROS production in activated macrophage photoreceptor cells.
| 100 tests | ・Photo-oxidation Resistant DCFH-DA Dye ・Loading Buffer (10x) |
100 nmol×1 1.0 ml×1 |
|---|
Description
Reactive oxygen species (ROS) are mainly produced in mitochondria during ATP synthesis. ROS plays an essential role in signaling pathways and the immune system, but excess ROS is associated with diseases and cellular senescence. DCFH-DA is widely used for ROS detection, but it has some limitations, such as weak fluorescence signals in cells, leakage from cells after staining, and photo-oxidation phenomena during observation1).
The dye that is employed in this kit allows ROS detection with higher sensitivity than DCFH-DA; It does not leak from cells because the fluorescent dye can immobilize protein via a chemical bond, and it is resistant to photo-oxidation compared with DCFH-DA. Moreover, the Loading Buffer in the kit maintains cellular health during assays.
1) M. Afzal, et al.,"Method to overcome photoreaction, a serious drawback to the use of dichlorofluorescin in evaluation of reactive oxygen species" BBRC, 2003, 304, 619–624.
Manual
Technical info
High data variability, high fluorescent background, and self-oxidation by observation light are the major challenges in ROS detection. Especially the self-oxidation may cause a false response, however Dojindo Laboratories' ROS Assay Kit -Photo-oxidation Resistant DCFH-DA- can solve these problems perfectly.
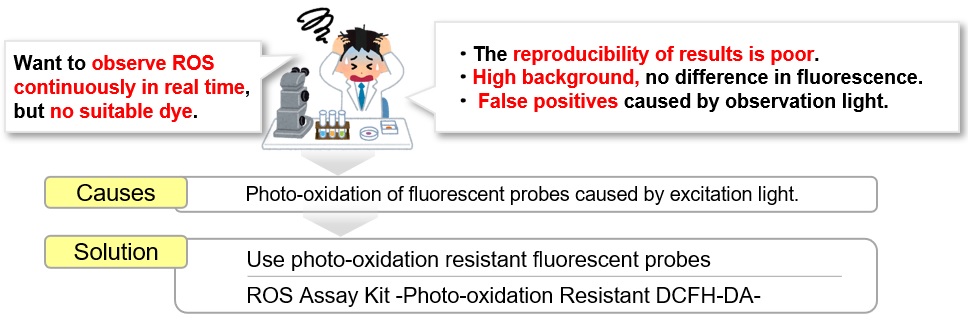
Reaction Selectivity for Reactive Oxygen Species
Photo-oxidation Resistant DCFH-DA shows the same reaction selectivity to reactive oxygen species (ROS) as DCFH-DA.
It also has the same fluorescence spectrum as DCFH-DA (λex: 505 nm, λem: 525 nm), so it can be detected at the same excitation and fluorescence wavelengths.
Comparison with Existing Reagents
A comparison of Dojindo Laboratories' probe and other probes for ROS detection.
| Dojindo Laboratories | Company T | |||
|---|---|---|---|---|
| Code | R253 | R252 | - | - |
| Product Name |
ROS Assay Kit |
Probe D | Probe C | |
|
Photo-oxidation |
★★★★★ |
- |
- |
- |
| Cell fixation |
★★★★★ |
- |
- |
★★★ |
|
Sensitivity |
★★★★ |
★★★★★ |
★★★ |
★★★ |
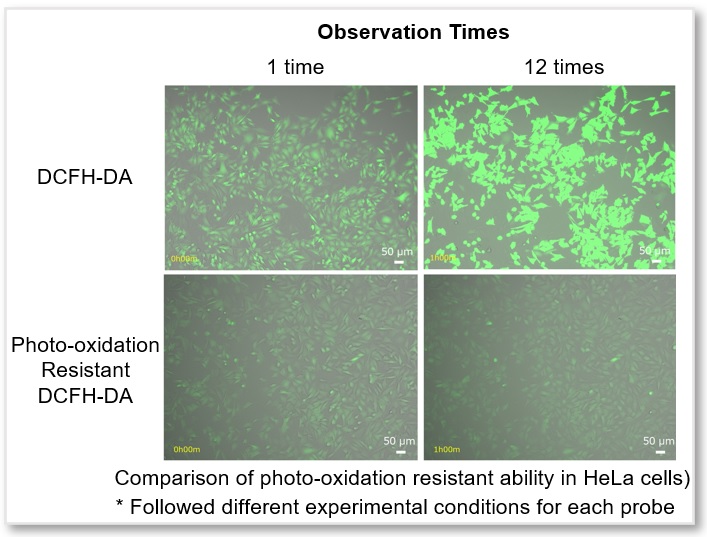
Time-lapse Imaging: Reduction of Photo-oxidation by Observation Light
Unstimulated HeLa cells were stained with regular DCFH-DA or Photo-oxidation Resistant DCFH-DA and observed with excitation light every 5 minutes for simultaneously fluorescence imaging. The results showed that the fluorescence intensity of DCFH-DA increased as the probe was photo-oxidated with excitation light, while Photo-oxidation Resistant DCFH-DA significantly suppressed photo-oxidation during observation.
Time-lapse imaging
<Experimental Conditions>
Cell Line: HeLa (without stimulation)
Epifluorescence microscope (BZ-X800, KEYENCE)
GFP Filter: Ex = 450 - 490 nm, Em = 500 - 550 nm
Time interval: 5 min.
Exposure time: 1.5 sec.
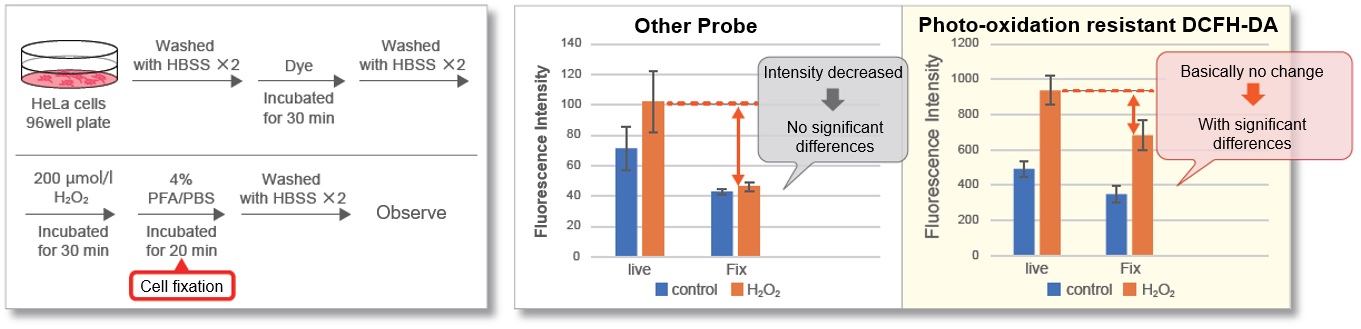
Cell Fixation after Staining
Most intracellular probes leak out cells after cell fixation. This is one of the reasons why ROS detection in fixed cells is more difficult than in live cells. After adding hydrogen peroxide to HeLa cells stained with photo-oxidation resistant DCFH-DA, the cells were fixed with PFA and compared with control cells. As a result, photo-oxidation resistant DCFH-DA showed a high retention ability and was able to maintain the difference from the control cells after cell fixation.
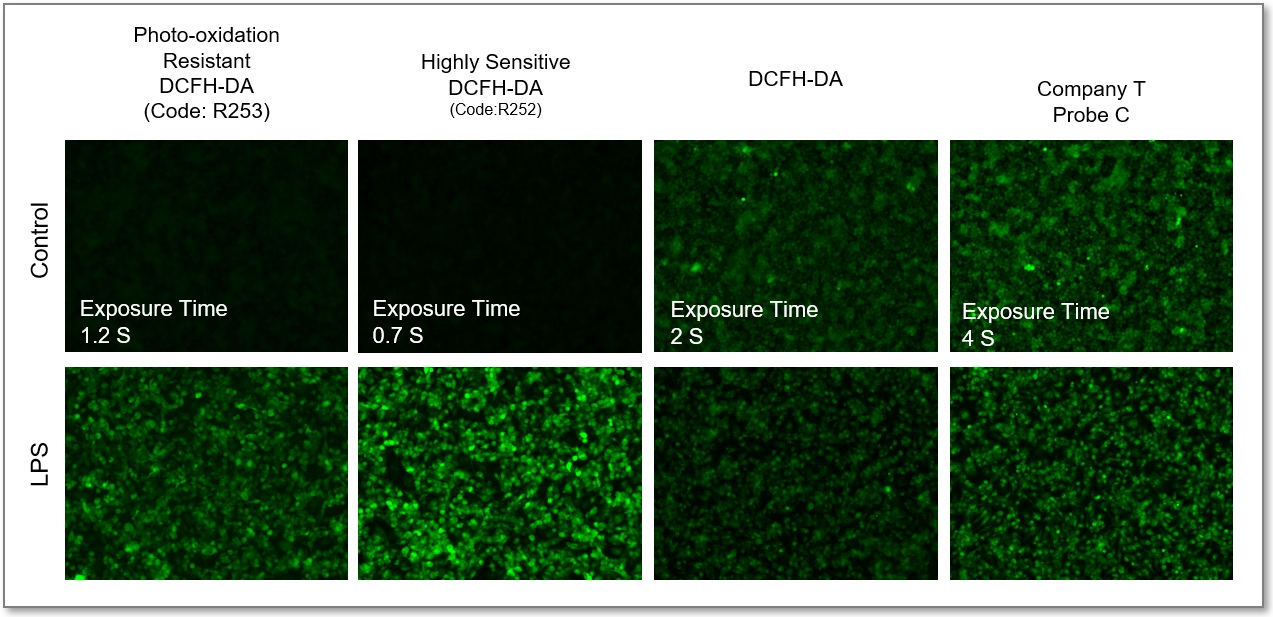
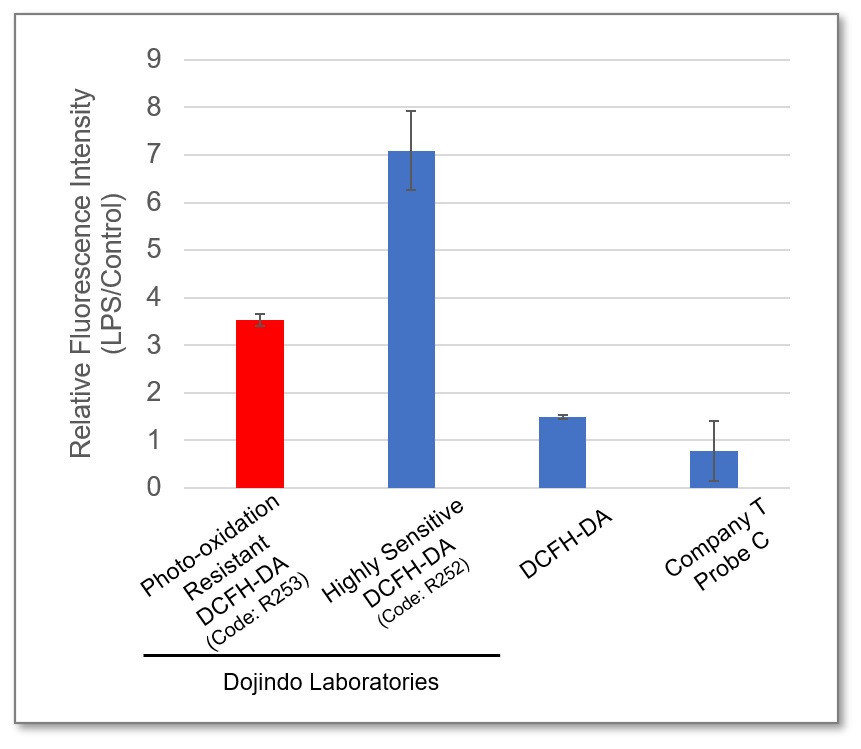
Comparison of Fluorescent Sensitivity
In Lipopolysaccharide (LPS) treated RAW 264.7 cells, after being stained with regular DCFH-DA, Highly Sensitive DCFH-DA, or Photo-oxidation Resistant DCFH-DA, the intracellular ROS level was compared. The results showed that the Dojindo Laboratories' probes could detect intracellular ROS with higher sensitivity.
① Fluorescent Microscope
*Different optimum observation conditions for each probe.
GFP Filter: Ex = 450 - 490 nm, Em = 500 - 550 nm)
② Plate Reader
Ex = 490 - 520 nm, Em = 510 - 540 nm
③ Flow Cytometer
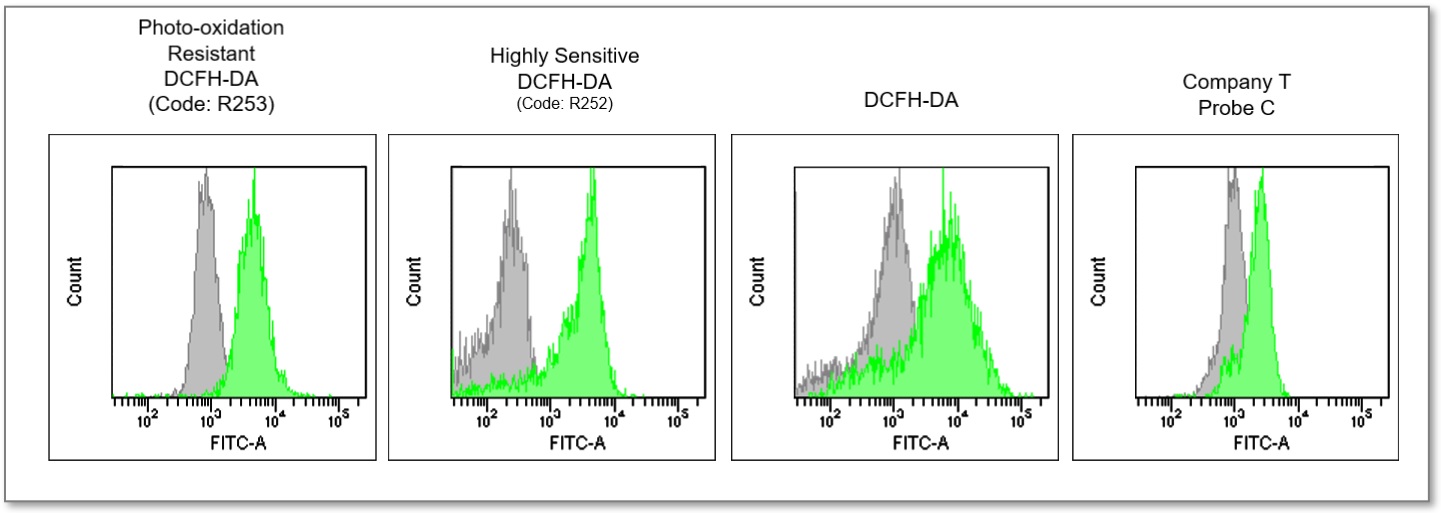
Ex = 488 nm, Em = 515 - 545 nm
Application Data: Simultaneous Detection of ROS in LPS-treated macrophages
In ROS Assay Kit -Photo-oxidation Resistant DCFH-DA- stained RAW264.7 cells, after LPS (Lipopolysaccharide)-treated, the intracellular ROS level was simultaneously observed with a fluorescence microscope. The fluorescence intensity graph showed that, after 10 hours of LPS-treated, the intracellular ROS level was significantly increased.
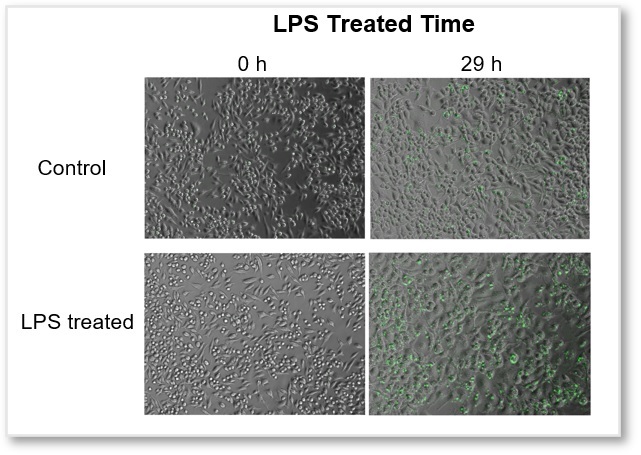
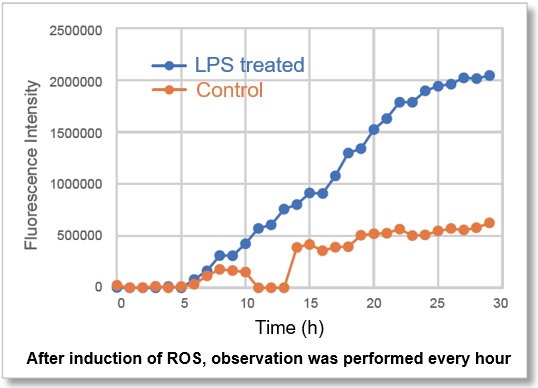
Video
Intracellular ROS(Photo-oxidation Resistant DCFH-DA Dye): GFP Filter (Ex = 450 - 490 nm, Em = 500 - 550 nm)
(1) RAW264.7 cells (2×105 cells/ml) in DMEM (supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin) were seeded on a 96-well black clear-bottomed plate.
(2) Incubated overnight at 37℃ in 95% air/5% CO2.
(3) The medium was removed, and the cells were washed twice with HBSS, then added Photo-oxidation Resistant DCFH-DA Dye Working Solution.
(4) Incubated for 30 mins at 37℃ in 95% air/5% CO2.
(5) Removed working solution, washed cells twice with HBSS, and DMEM containing LPS (500 ng/ml) was added to the cells.
(6) The cells were observed every hour for 30 hours under an epifluorescence microscope (BZ-X800, KEYENCE).
(7) Fluorescence signals were quantified.
Application Data: Co-staining with Mitochondrial Marker Tom 20
After adding hydrogen peroxide, HeLa cells were stained with Photo-oxidation resistant DCFH-DA and mitochondrial marker Tom 20. As a result, the intracellular ROS level and mitochondrial morphology were clearly observed at the same time, which was very difficult for the existing ROS probe.

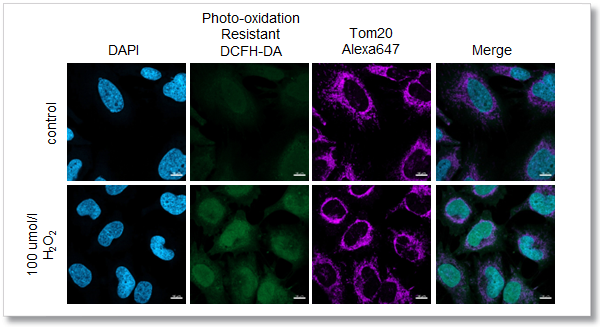
<Experimental Conditions>
Confocal laser microscope
(Blue) DAPI: Ex = 405 nm, Em = 450-495 nm
(Green) Photo-oxidation Resistant DCFH-DA: Ex = 488 nm, Em = 500-550 nm
(Deep Red) Alexa Fluor 647: Ex = 633 nm, Em = 640-700 nm
Scale bar: 10 µm
Application Data: High-content live cell imaging of ROS production in activated macrophages
Immune cells play a role in maintaining a normal body by eliminating foreign substances through the inflammatory response.
Monocytes stimulated by foreign substances produce Reactive Oxygen Species(ROS) in mitochondria, differentiate into activated macrophages, and decompose foreign substances. However, excessive or chronic inflammation can potentially lead to autoimmune diseases, allergies, diabetes, and neurodegenerative disorders such as Alzheimer's disease. In this context, ROS plays a pivotal role, and monitoring ROS can help researchers understand the extent of an inflammatory response, as well as evaluate the effectiveness of drugs designed to modulate this response.
Lipopolysaccharide (LPS) stimulated macrophages could phagocytose dead cells. The inflammatory response in LPS induced macrophages was evaluated with ROS as an indicator. ROS was detected with ROS Assay Kit -Photo-oxidation Resistant DCFH-DA- on Nikon inverted microscope(Ti2-E). In addition, changes in ROS per cell were detected over time using volume contrast images.
Observation of cell morphology & cell counting
LPS stimulation increased the cell area per cell (Figure 1A) and inhibited the increasing in cell number compared to control cells (Figure 1B).
ROS Assay
The fluorescence intensity per cell was calculated in the entire field of view. 21 hours after the start of imaging, the fluorescence intensity of 1,000 ng/ml of LPS was higher than that of 500 ng/ml, and the result shows that higher LPS concentration has higher amount of ROS produced per cell (Figure 2). Furthermore, time-lapse imaging showed an increase in cell number due to cell division, a decrease in cell number due to phagocytosis of dead cells by macrophages, and an increase in ROS (Figure 3).
With conventional ROS detection reagents, highly reproducible experiments were difficult to carry out due to auto-oxidation caused by excitation light. The ROS detection reagent from DOJINDO LABORATORIES used in this experiment is a reagent whose dyes exhibit reduced auto-oxidation due to excitation light. Fluorescence was detected specifically in the area where ROS was produced in the cell, and background noise was low, enabling highly accurate analysis. In addition, the intracellular residence of the dye is high, and high-intensity fluorescence was detected even in images taken 48 hours after the start of imaging, enabling analysis of ROS over a long period of time.
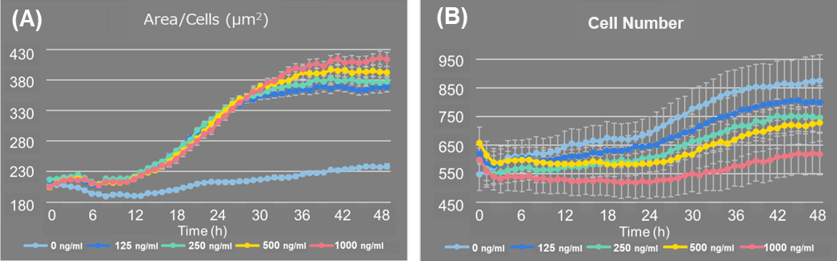
Fig 1. Changes in cell number and cell area per cell after adding LPS
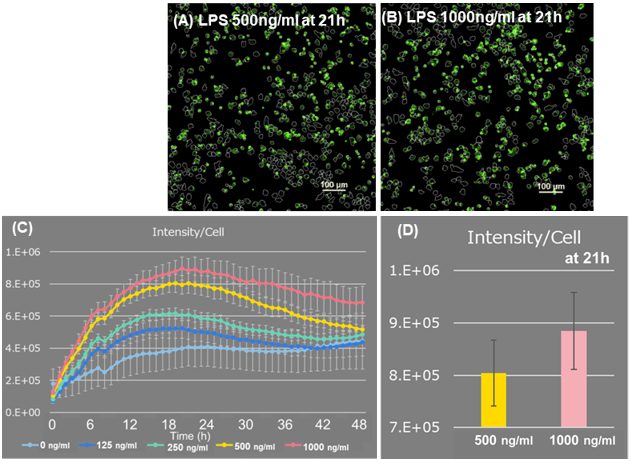
Figure 2. Fluorescence intensity analysis of ROS Assay
(A, B) 21 hours after adding LPS Scale bar: 100 μm
(C, D) ROS fluorescence intensity per cell
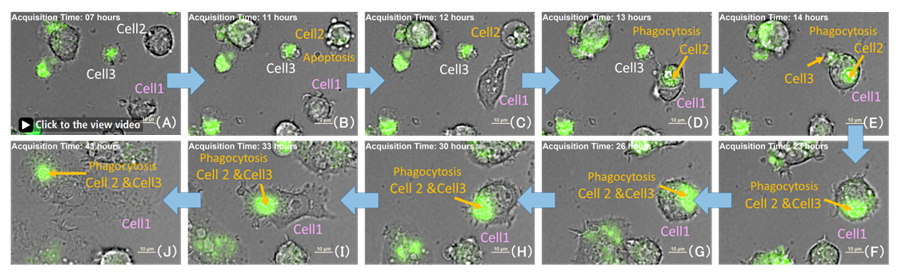
Figure 3. Phagocytosis of dead cells by activated macrophages
Time-lapse imaging of LPS 500 ng/ml well。Green: ROS, Scale bar: 10 μm
Cell 2, Apoptosis cells (B) After 1 hour showed dead cell morphology(C).
Cell 1 phagocytosed Cell 2 and Cell 3(dead cells) and ROS level increased(C-F)
*This experiment is corrected with cell number using Volume Contrast(VC) imaging system.
VC imaging can construct a fluorescence-like phase distribution image from three brightfield images with different focal planes, making it easy to distinguish between the background and the cell regions. This makes cell counting without any cell damage.
<Instrument>
High-content Analysis(HCA) Microscope system
(Nikon https://www.microscope.healthcare.nikon.com/en_EU/)
*About the detail protocol and analysis please refer to:
Nikon ”APPLICATION NOTE: High-content live cell imaging of ROS production in activated macrophages”
References
| No. | Sample | Instrument | Other target or Reagents | Reference |
|---|---|---|---|---|
| 1 | HaCaT, D66H cells | Fluorescence microscope *Nuclear staining: Hoechst33342 |
SOD, Lipid peroxidation, GSH/GSSG | Y. Chen, Z. Wang, Y. Song, N. Chen, J. Guo, W. Liu, K. Guo, X. Ling and L. Zhang,"4‐octyl itaconate improves the viability of D66H cells by regulating the KEAP1‐NRF2‐GCLC/HO‐1 pathway", J Cell Mol Med., 2022, doi: 10.1111/jcmm.17708. |
| 2 | Nucleus pulposus cells | Fluorescence microscope *Nuclear staining: Hoechst33342 |
Mitochondrial membrane potential (MT-1) | K. Suyama, D. Sakai, S. Hayashi, N. Qu, H. Terayama, D. Kiyoshima, K. Nagahori and M. Watanabe,"Bag-1 Protects Nucleus Pulposus Cells from Oxidative Stress by Interacting with HSP70", Biomedicines., 2023, doi: 10.3390/biomedicines11030863. |
| 3 | HCC cells | Fluorescence microscope | GSH/GSSG, γH2AX, Cell cycle | H. Liu, W. Zhang, L. Jin, S. Liu, L. Liang and Y. Wei,"Plumbagin Exhibits Genotoxicity and Induces G2/M Cell Cycle Arrest via ROS-Mediated Oxidative Stress and Activation of ATM-p53 Signaling Pathway in Hepatocellular Cells", Int. J. Mol. Sci., 2023, doi: 10.3390/ijms24076279. |
| 4 | Human cord blood cd34+ cells | Flow Cytometer | γH2AX | M. Sakurai, K. Ishitsuka, T. Ito, A. C. Wilkinson, T. Kimura, E. Mizutani, H. Nishikii, K. Sudo, H. J. Becker, H. Takemoto, T. Sano, K Kataoka, S. Takahashi, Y. Nakamura, D. G. Kent, A. Iwama, S. Chiba, S. Okamoto, H. Nakauchi and S. Yamazaki, "Chemically defined cytokine-free expansion of human haematopoietic stem cells", Nature, 2023, doi:10.1038/s41586-023-05739-9. |
Q & A
-
Q
How many samples can be measured with this kit?
-
A
Please refer the follows:
・ 96-well plate:1 plate
・ ibidi 8-well plate:6 plates
・ 35 mm dish:5 dishes
・ 6-well plate:5 wells
-
Q
Do you have an example of an experiment that would be a positive control?
-
A
The second application data in the product manual is an example of ROS detection in hydrogen peroxide treated HeLa cells which can be considered as a positive control. Please refer to protocol steps 1 - 5 in the second application data.
-
Q
Can Working Solution be prepared with anything other than Loading Buffer?
-
A
In order to decrease the cell damage, using Loading buffer of HBSS to prepare Working solution is recommended. If you need medium to prepare working solution, please use serum-free medium.
-
Q
Can PBS be used instead of HBSS for washing after staining?
-
A
To decrease cell damage, HBSS is recommended. If you don't have HBSS, please use a medium to wash the cells.
-
Q
When using a flow cytometer, at what stage should the cells be dissociated?
-
A
After step 6 in the general protocol (Wash cells after ROS-inducing treatment ), use trypsin to dissociate the cells, add medium to stop trypsin reaction and then wash the cells once with HBSS, centrifuge again, and resuspend the cells with HBSS as a sample.
Handling and storage condition
| Store at 0-5℃ oC |