ROS Assay Kit -Highly Sensitive DCFH-DA-
ROS Detection
- High-sensitivity detection of total ROS
- Detectable using fluorescence microscope, plate reader, and flow cytometer
-
Product codeR252 ROS Assay Kit -Highly Sensitive DCFH-DA-
| Unit size | Price | Item Code |
|---|---|---|
| 100 tests | $198.00 | R252-10 |
| 100 tests | ・Highly Sensitive DCFH-DA Dye ・Loading Buffer (10x) |
10 ×l×1 1.0 ml×1 |
|---|
Description
Reactive oxygen species (ROS) are primarily produced in mitochondria during ATP synthesis. ROS play an essential role in signaling pathways and the immune system, whereas increasing ROS is associated with diseases and cellular senescence. Recent studies suggested that ferroptosis is a new type of cell death characterized by iron dependency and increasing ROS. Thus, ROS detection has been attracting considerable interest in ferroptosis research. DCFH-DA is widely used for ROS detection, but it has some limitations like weak fluorescence signals and high background. Dojindo’s ROS Assay Kit -Highly Sensitive DCFH-DA- overcomes these limitations. The dye employed in the kit allows ROS detection with higher sensitivity than DCFH-DA. Moreover, the Loading Buffer included in this kit maintains cellular health during assays.
Manual
Technical info
A comparison of Dojindo Laboratories' probe and other probes for ROS detection.
| Dojindo Laboratories | Company T | |||
|---|---|---|---|---|
| Code | R252 | R253 | - | - |
| Product Name |
ROS Assay Kit |
Probe D | Probe C | |
|
Photo-oxidation |
- |
★★★★★ |
- |
- |
| Cell fixation |
- |
★★★★★ |
- |
★★★ |
|
Sensitivity |
★★★★★ |
★★★★ |
★★★ |
★★★ |
Comparison the Highly Sensitive DCFH-DA with DCFH-DA
The selectivity of these probes for ROS
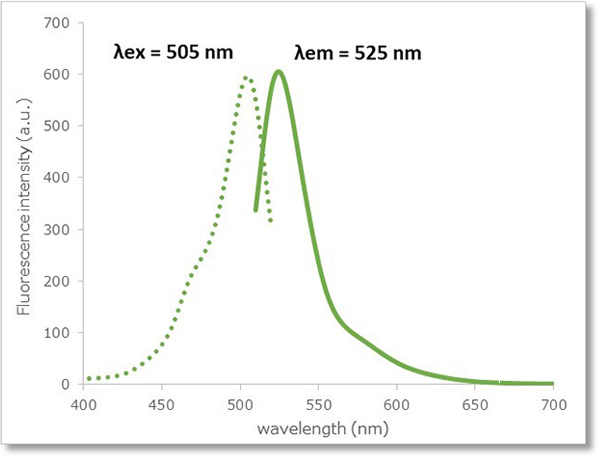
The reactivity of the Highly Sensitive DCFH-DA for ROS is similar to the reactivity of 2’-7’ dichlorofluorescein diacetate (DCFH-DA). The Highly Sensitive DCFH-DA also has similar fluorescence characteristics (λex: 505 nm, λem: 525 nm) to DCFH-DA. Therefore, ROS is detectable at the same excitation/fluorescence wavelength.

Comparison of detection sensitivity
Hydrogen peroxide (H2O2)-treated HeLa cells (1×104 cells/ml) were stained with DCFH-DA or the ROS Assay Kit-Highly
Sensitive DCFH-DA, and the detectability of intracellular ROS was compared between two detection kits. As a result, the ROS Assay Kit-Highly Sensitive DCFH-DA in high-sensitivity detection of intracellular ROS was better than DCFH-DA.
① Detection using fluorescent microscope
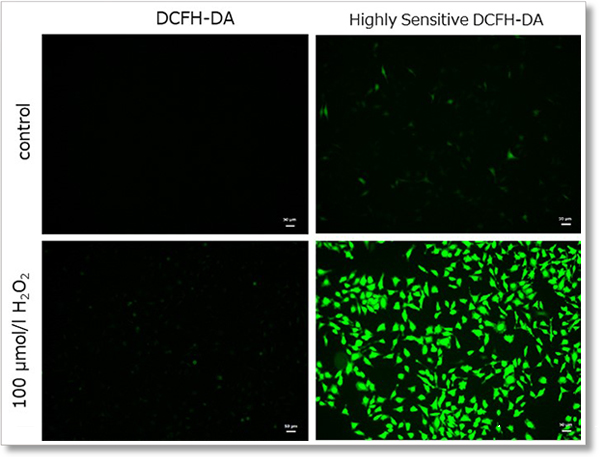
Detection conditions
Ex. 488 nm / Em. 500 - 560 nm
Cell type:HeLa cells
(scale bar:50 µm)
② Detection using microplate reader
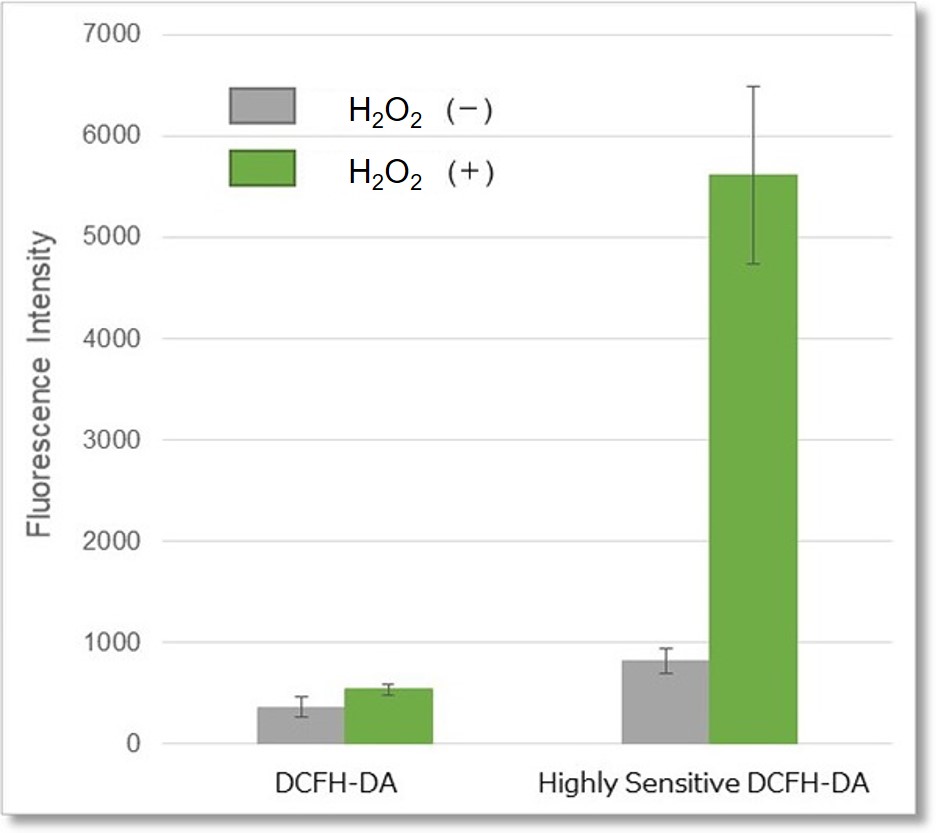
Detection conditions
Ex. 490 - 520 nm / Em. 510 - 540 nm
Cell type:HeLa cells
③ Detection using flow cytometer
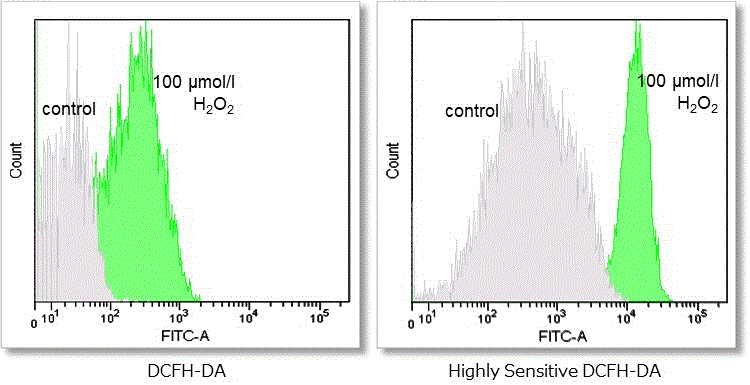
Detection conditions
FITC laser gain:215 V
Cell type:HeLa cells
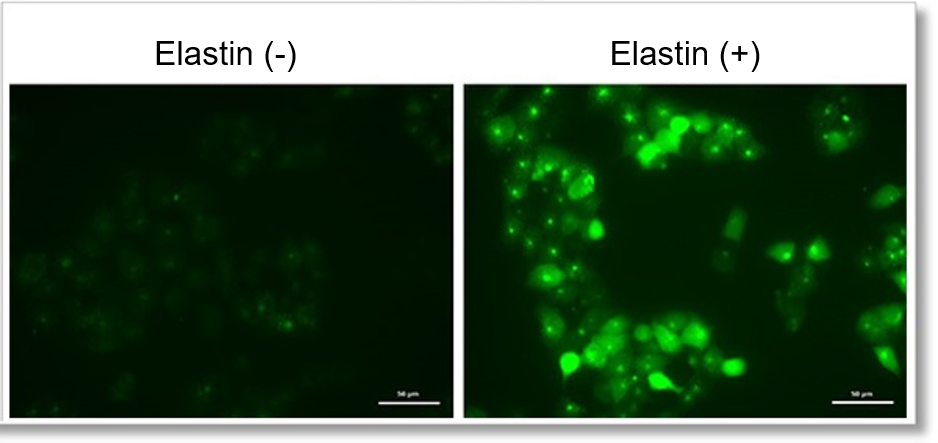
Detection of endogenous ROS in A549 cells treated with Elastin.
Elastin treatment, which inhibits cystine/glutamate antiporter (xCT), is known to induce iron-dependent cell death, also known as ferroptosis. The imaging of changes in intracellular ROS revealed that elastin treatment increased the generation of ROS in elastin-treated A549 cells.
|
|
Detection conditions
Intracellular ROS(Highly Sensitive DCFH-DA Dye): Ex. 488 nm / Em. 500 - 560 nm
Potocol
1. A549 cells (1×104 cells/ml) in DMEM (supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin) were seeded on ibidi 8-well plates.
2. The cells were cultured overnight in an incubator set at 37℃ and equilibrated with 95% air and 5% CO2.
3. Medium was removed and DMEM containing Elastin (50 μmol/l) was added to the cells.
4. The cells were cultured overnight as in step 2.
5. After removing the supernatant, the cells were washed twice with HBSS and treated with Highly Sensitive DCFH-DA working solution.
6. The cells were incubated for 30 minutes as in step 2.
7. Supernatant was removed, and cells were washed twice with HBSS and refilled with HBSS. The cells were observed under a fluorescence microscope.
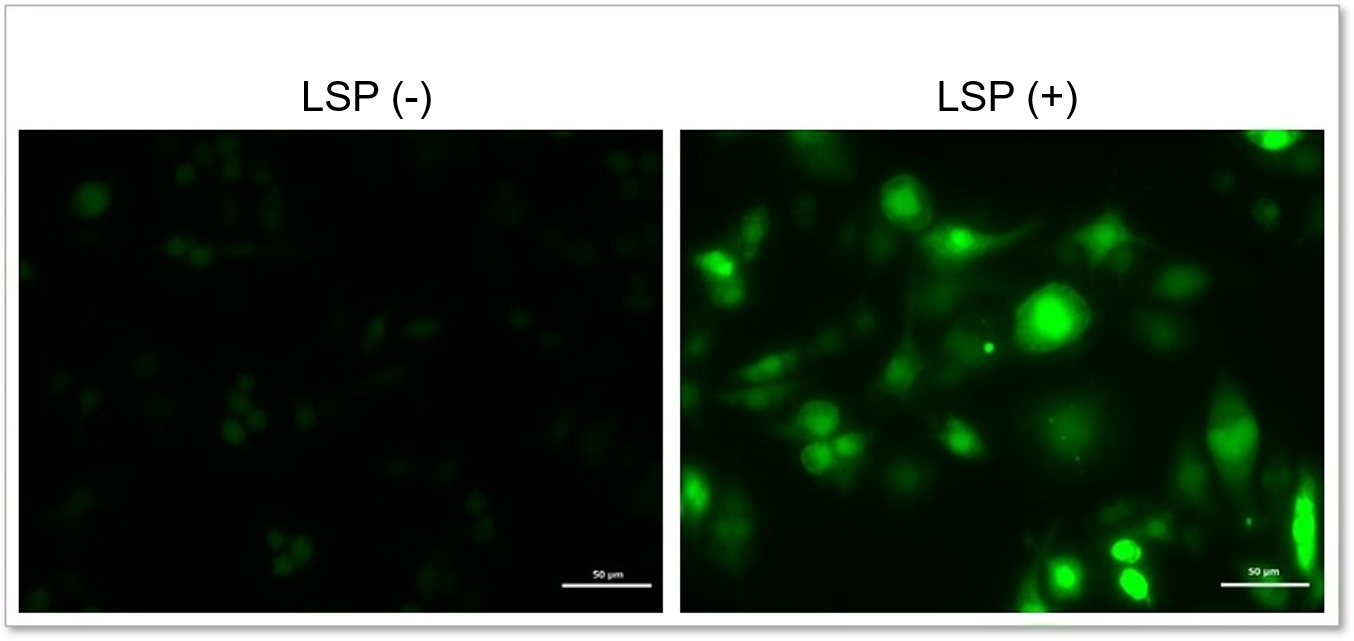
Detection of endogenous ROS in RAW264.7 cells treated with lipopolysaccharide (LPS).
In LPS-treated RAW264.7 cells, the imaging of changes in intracellular ROS revealed that LPS treatment increased the generation of ROS.
|
|
Detection conditions
Intracellular ROS(Highly Sensitive DCFH-DA Dye): Ex. 488 nm / Em. 500 - 560 nm
Potocol
1. RAW264.7 (3×104 cells/ml) in DMEM (supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin) were seeded on ibidi 8-well plates.
2. The cells were cultured overnight in an incubator set at 37℃ and equilibrated with 95% air and 5% CO2.
3. Medium was removed and DMEM containing LPS (500 ng/ml) was added to the cells.
4. The cells were cultured for 20 hours as in step 2.
5. After removing the supernatant, the cells were washed twice with HBSS and treated with Highly Sensitive DCFH-DA working solution.
6. The cells were incubated for 30 minutes as in step 2.
7. Supernatant was removed, and cells were washed twice with HBSS and refilled with HBSS. The cells were observed under a fluorescence microscope.
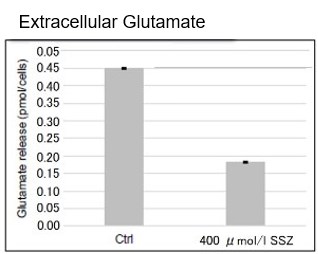
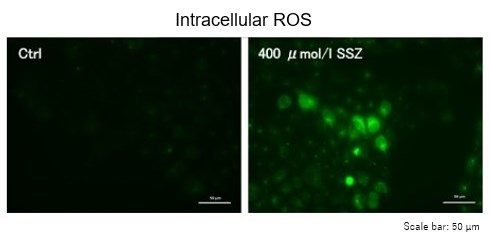
Sulfasalazine Alters Intracellular Metabolites and Increases ROS in A549 Cells
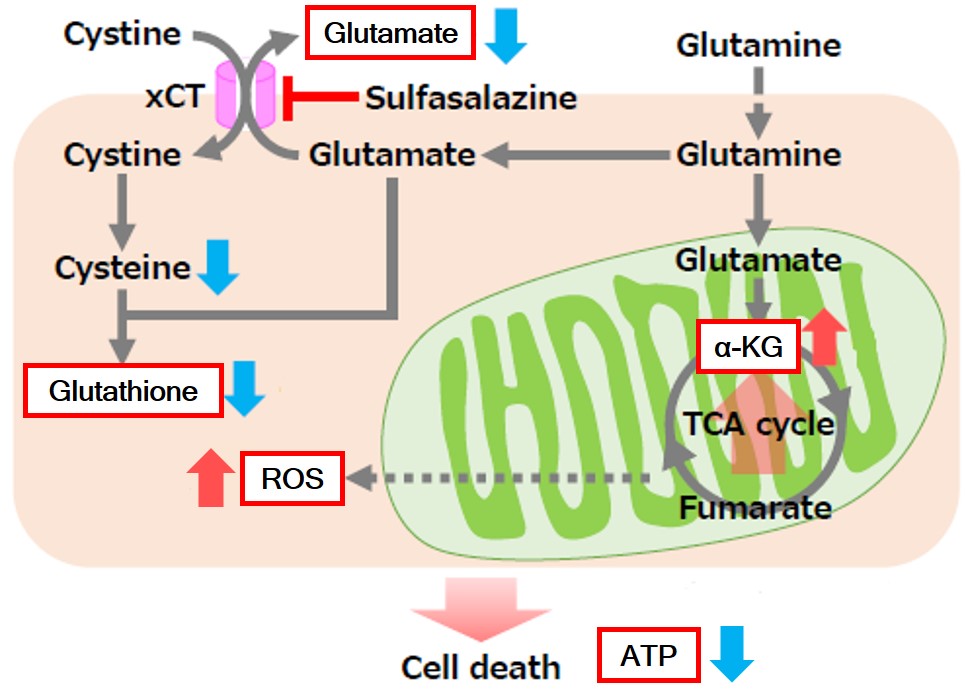 |
After addition of sulfasalazine (SSZ), a known inhibitor of cystine/glutamate transporter (xCT), to A549 cells, we observed changes in intracellular glutathione (GSH), ATP, α-ketoglutarate (α-KG), ROS and glutamate release. The results indicated that the addition of SSZ
|
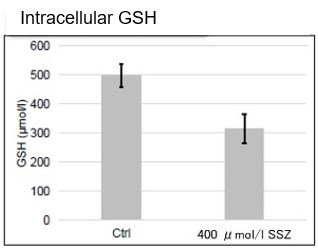 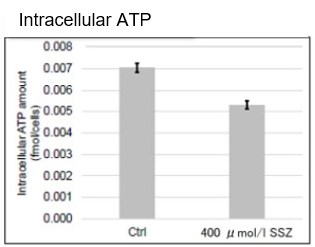 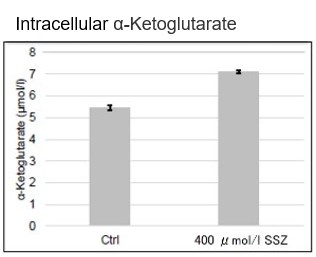   |
|
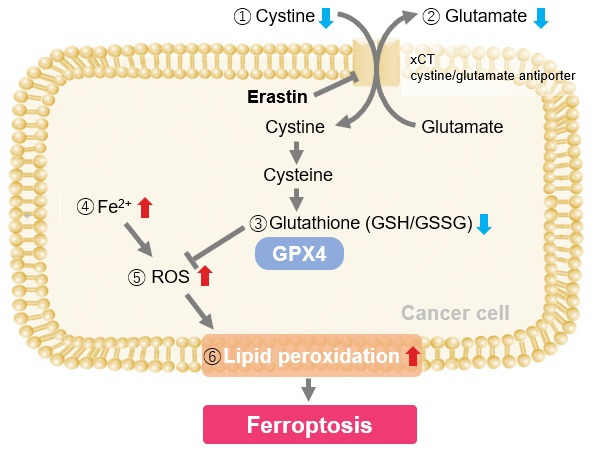
Induction of Ferroptosis by Erastin
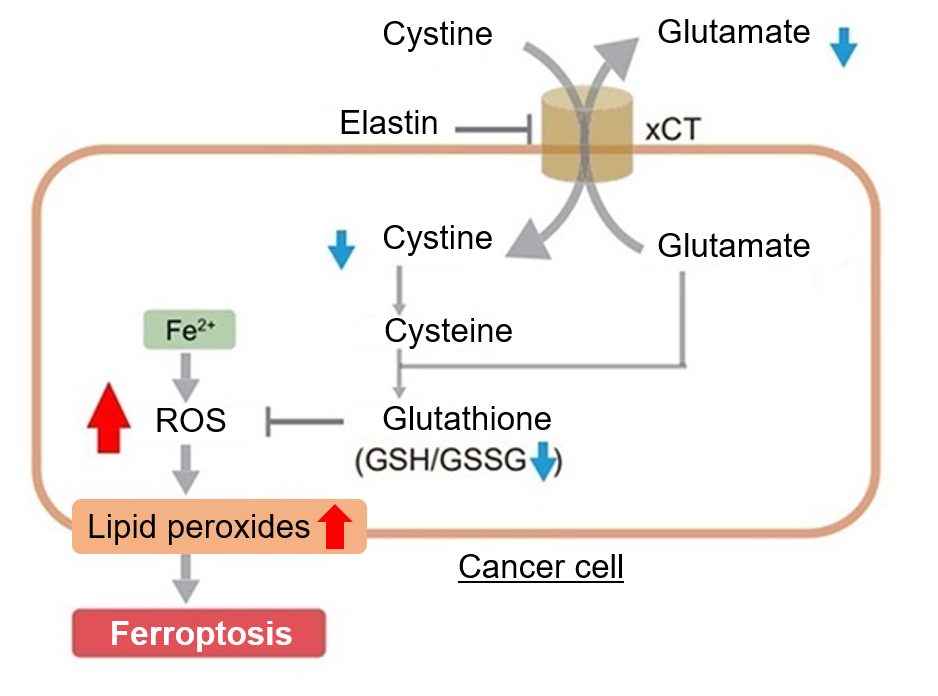
Erastin is a known inducer of ferroptosis. By inhibiting the cystine transporter (xCT), erastin inhibits the uptake of cystine. Cystine is the raw material for GSH. Therefore, Erastin ultimately decreases the amount of GSH. Decreased GSH then results in lipid peroxide accumulation and induction of ferroptosis.
The following experimental examples show changes in each aforementioned index as a consequence of erastin stimulation. Measurements are made using Dojindo reagents.
Using erastin-treated A549 cells, we measured intracellular Fe2+, ROS, lipid peroxide, glutathione, glutamate release into the extracellular space, and cystine uptake. As a result, inhibition of xCT by elastin was observed and also the release of glutamate and uptake of cystine were decreased. Furthermore, elastin treatment decreased intracellular glutathione while it increased intracellular Fe2+ , ROS, and lipid peroxides.

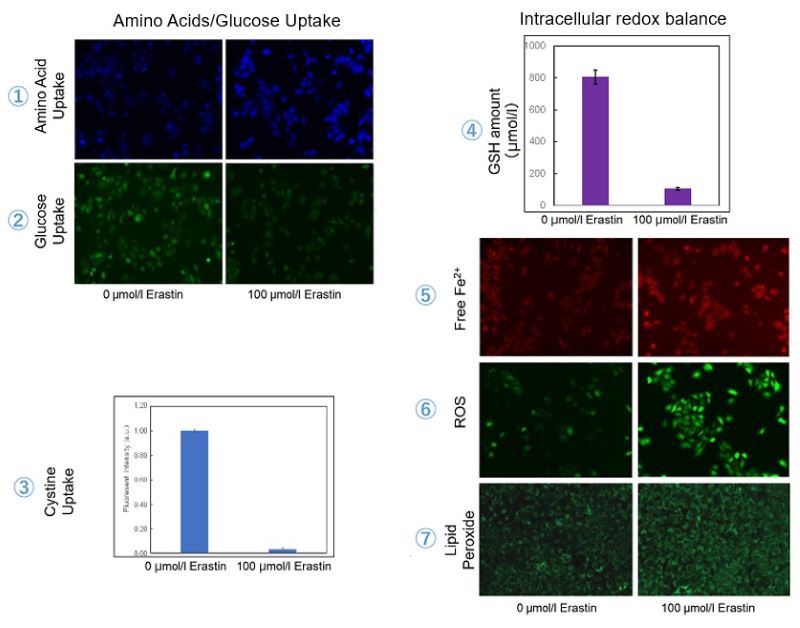
| 1 Amino Acid Uptake | : Amino Acid Uptake Assay Kit (Code: UP04) |
| 2 Glucose Uptake | : Glucose Uptake Assay Kit-Green (Code: UP02) |
| 3 Cystine Uptake | : Cystine Uptake Assay Kit (Code: UP05) |
| 4 Intracellular glutathione | : GSSG/GSH Quantification Kit (Code: G257) |
| 5 Intracellular labile Fe | : FerroOrange (Code: F374) |
| 6 Intracellular total ROS | : ROS Assay Kit -Highly Sensitive DCFH-DA- (Code: R252) |
| 7 Lipid Peroxides | : Liperfluo (Code: L248) |
Cell Line: A549
Incubation Conditions: 100 μmol/l Erastin/MEM, 37℃, 3h
Fluorescence Property
References
| No. | Sample | Instrument | Reference (Link) |
|---|---|---|---|
| 1) |
Cell |
Fluorescence microscope | S. Kato, "Effects of platinum‑coexisting dopamine with X‑ray irradiation upon human glioblastoma cell proliferation", Hum. Cell, 2021, doi:10.1007/s13577-021-00591-3. |
| 2) |
Cell |
Fluorescence microscope | K. Kanno, T. Sakaue, M. Hamaguchi, K. Namiguchi, D. Nanba, J. Aono, M. Kurata, J. Masumoto, S. Higashiyama, H. Izutani, "Hypoxic Culture Maintains Cell Growth of the Primary Human Valve Interstitial Cells with Stemness", Int. J. Mol. Sci. 2021, doi:10.3390/ijms221910534. |
| 3) |
Cell |
Flow Cytometer | C. Fang, L. Wu, M. Zhao, T. Deng, J. Gu, X. Guo, C. Li, W. Li, X. Zeng, "Periodontitis Exacerbates Benign Prostatic Hyperplasia through Regulation of Oxidative Stress and Inflammation", Oxid. Med. Cell. Longevity, 2021, doi:10.1155/2021/2094665. |
| 4) | Bacteria (Synechococcus elongatus PCC7942) |
Plate Reader | M. Tamoi and S. Shigeoka, "CP12 Is Involved in Protection against High Light Intensity by Suppressing the ROS Generation in Synechococcus elongatus PCC7942", Plants, 2021, doi:10.3390/plants10071432. |
| 5) | Cell SW982 |
Fluorescence microscope | S. Xia, Z. Ma, B. Wang, F. Gao, C. Yi, X. Zhou, S. Guo, L. Zhou, "In vitro anti-synovial sarcoma effect of diallyl trisulfide and mRNA profiling", Gene, 2021, doi:10.1016/j.gene.2021.146172. |
| 6) | Cell BRL3A Cell |
Fluorescence microscope | Z. Luo, Q. Gao, H. Zhang, Y. Zhang, S. Zhou, J. Zhang, W. Xu, J. Xu, " Microbe-derived antioxidants attenuate cobalt chloride-induced mitochondrial function, autophagy and BNIP3-dependent mitophagy pathways in BRL3A cells", 2022, doi:10.1016/j.ecoenv.2022.113219. |
Q & A
-
Q
How many samples can be measured with one kit?
-
A
Please refer to the following for the number of samples that can be measured.
96-well plate: 1 plate
ibidi 8-well plate: 6 plates
35 mm dish: 5 dishes
6-well plate: 5 wells
-
Q
Can you show me any example of a positive control?
-
A
Please refer to the technical manual for the example of detection using H2O2-treated HeLa cells.
-
Q
May I use PBS instead of HBSS to wash cells after staining?
-
A
We recommend using HBSS to reduce cell damage. If you do not have HBSS, please rinse them with a culture medium.
-
Q
May I use anything other than the Loading Buffer for the preparation of the Highly Sensitive DCFH-DA working solution?
-
A
To reduce cell damage resulting from staining, we recommend using the Loading Buffer supplied with the kit; however, you can also use Hanks’ HEPES or HBSS for the preparation. Please use a serum-free medium if you prepare the working solution in a culture medium.
-
Q
Is there anything that I should be careful of when using a fluorescence microscope?
-
A
The Highly Sensitive DCFH-DA reacts with ROS and then becomes oxidized. Fluorescence is emitted after the oxidation of dye. When exposed to the excitation light for a long period, the dye becomes oxidized, which then causes the background to rise. Therefore, use bright field illumination, adjust the focus, and then acquire fluorescence images.
Handling and storage condition
| -20℃ | |
|
Danger / harmful symbol mark |

|
|---|---|