FerroOrange
Intracellular Iron Measurement
-
Product codeF374 FerroOrange
-
CAS No.-
| Unit size | Price | Item Code |
|---|---|---|
| 1 tube | $158.00 | F374-10 |
| 3 tube | $365.00 | F374-12 |
*1 tube (24 µg) can be used for 17 assays at 35 mm dish (final concentration 1 µmol/L).
Description
Iron is the most abundant transition metal element within organisms, and it participates in various physiological activities. Recently, free iron in living cells has attracted attention because its high reactivity may be related to cellular damage or death. Free iron exists in its stable redox states, namely ferrous ion (Fe2+) and ferric ion (Fe3+). In living cells, understanding the behavior of Fe2+ is considered more important than understanding that of Fe3+ because of the intracellular reductive environment, metal transporters, and the water solubility of Fe2+. FerroOrange has no chelating ability. FerroOrange and Fe2+ react irreversibly, which is different from the detection principle of calcium-iron probes such as Fluo-3.
In 2012, Ferroptosis was proposed as one of new cell deaths. Ferroptosis is studied as non apoptotic cell death caused by accumulation of iron ion-dependent lipid peroxide. FerroOrange is a novel fluorescent probe that enables live-cell fluorescent imaging of intracellular Fe2+, used in ferroptosis research.
Ferroptosis Analysis Products
| Product Name | Target | Ditection Properties |
|---|---|---|
| FerroOrange | Intracellular Fe2+ | Microscopy, Plate reader Ex: 543 nm / Em: 580 nm |
| Mito-FerroGreen | Mitochondrial Fe2+ | Microscopy Ex: 505 nm / Em: 535 nm |
| Liperfluo | Lipid Peroxide | Microscopy, FCM Ex: 488 nm / Em: 500-550 nm |
| Lipid Peroxidation Probe -BDP 581/591 C11- |
Lipid Peroxidation Process |
Microscopy, FCM, Plate reader |
| MDA Assay Kit | Malondialdehyde | Plate reader Fluorescence, Ex: 540 nm / Em: 590 nm Colorimetric, λ: 532 nm |
| Cystine Uptake Assay Kit | Cystine uptake | Plate reader Ex: 485 nm / Em: 535 nm |
| GSSG/GSH Quantification Kit | GSSG and GSH | Plate reader Colorimetric, λ: 405 nm |
Manual
Technical info
Experimental Example
HeLa cells treated with chelator of iron 2,2′-bipyridyl (Bpy) (100 μmol/L) or Ammonium iron (II) sulfate (100 μmol/L) were prepared. The change of intracellular Fe2+in HeLa cells was detected by the FerroOrange.
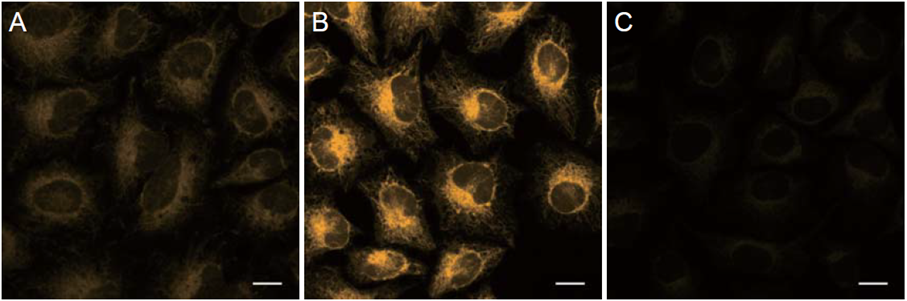
Ex/Em = 561 nm/570-620 nm, Scale bars 20 μm
A: Control
B: Ammonium iron (II) sulfate treated
C: Ammonium iron (II) sulfate and 2,2′-Bipyridyl (Bpy) treated
The fluorescence intensity of FerroOrange was increased in HeLa cells treated with Ammonium iron (II) sulfate compared with the findings in untreated cells; conversely, its fluorescence intensity was decreased in cells treated with Bpy.
Easy quantification by plate assay
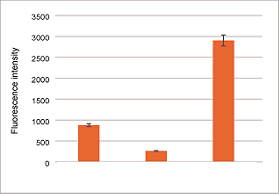
Ex/Em = 543 nm/ 580 nm
Left Control
Middle Ammonium iron (II) sulfate and 2,2′-Bipyridyl (Bpy) treated
Right Ammonium iron (II) sulfate treated
The change of intracellular Fe2+in HeLa cells was quantified by the FerroOrange.
High selectivity for Fe2+
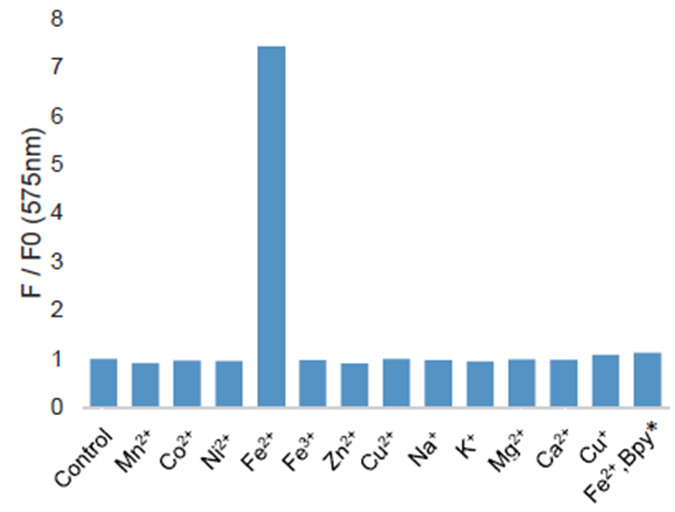
Ex/Em = 543 nm/ 580 nm
2 μL of 1 mmol/L FerroOrange and 2 μL of 10 mmol/L from each metal were added to 1 mL of 50 mmol/L HEPES Buffer (pH7.4). The fluorescence intensity was measured after the reaction, for 1hr, at room temperature.
Applications
FerroOrange is ready to use for a 96-well-plate-based high-content imaging of labile Fe(II) in living cells. In the following article, Dr. Hirayama etc. were able to conduct high-throughput screening of a chemical library containing 3399 compounds.
*FerroOrange is mentioned as “RhoNox-4” in the reference.
*FerroOrange was commercialized under the advisory of Dr. Hideko Nagasawa and Dr. Tasuku Hirayama (Gifu Pharmaceutical University).
In the following article, ferrous ions (Fe2+) were detected in mouse livers using FerroOrange (Ferroptosis research).
*mice were fed on methionine- choline deficient diet
For details about the experiment, please visit the reference below.
Co-staining with Each Organelle Dye Reagent
FerroOrange was co-stained with each organelle’s dye reagents.
HeLa cells were stained with organelle’s dye and washed.
Then, FerroOrange was added to the cells and cells were observed under the fluorescent microscope.
Co-staining with ER Staining Dye
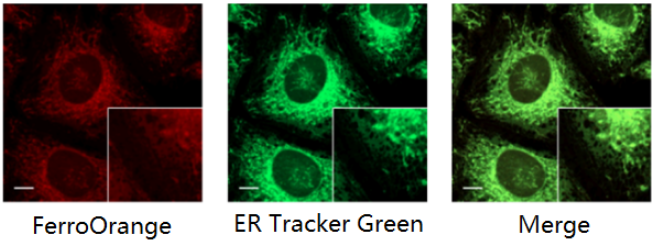
FerroOrange: Ex. 561 nm, Em. 570-620 nm
ER Tracker Green (ER Dye): Ex. 488 nm, Em. 510-555 nm
Scale bars: 10 µm
Co-staining with Mitochondrial staining Dye
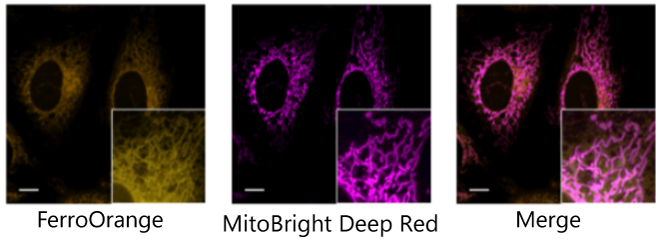
FerroOrange: Ex. 561 nm, Em. 570-620 nm
MitoBright Deep Red (Mitochondrial Dye): Ex. 640 nm, Em. 650-700 nm
Scale bars: 10 µm
Co-staining with Golgi Complex Staining Dye
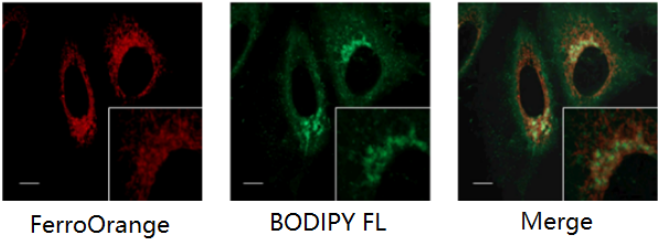
FerroOrange: Ex. 561 nm, Em. 570-620 nm
BODIPY FL (Golgi Complex Staining Dye): Ex. 488 nm, Em. 510-555 nm
Scale bars: 10 µm
Fruorescence property
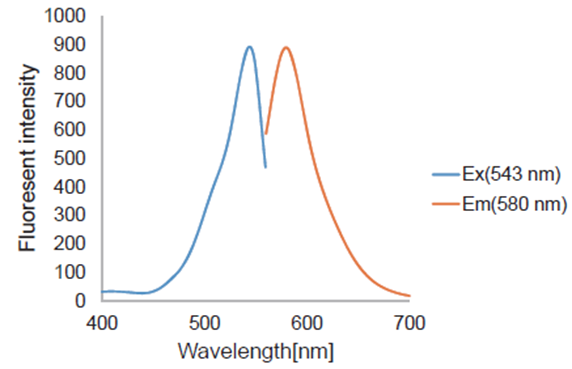
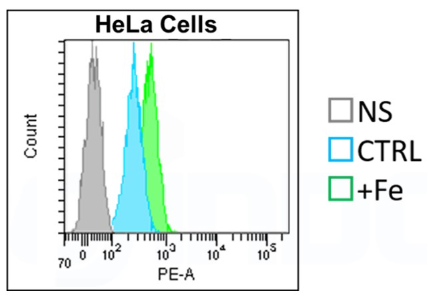
Quantitative Analysis by Flow Cytometer
・FerroOrange may affect staining intensity, depending on cell density and cell type.
*Please refer to Q&A “Is it necessary to wash cells after staining?”.
・Furthermore, a dye may leak out of the cells, depending on a medium change and a wash.
・For these reasons, you need to optimize when performing quantitative flow cytometry.
1. HeLa cells (1 x105 cells/well) in MEM (10% fetal bovine serum, 1% penicillin-streptomycin) were seeded on a 6 well plate and were cultured at 37oC in a 5% CO2 incubator overnight.
2. The cells were washed with serum-free medium (2 mL) three times. Then, serum-free medium (1 mL) was added to the cells.
3. 10 mmol/L Ammonium iron (II) sulfate (10 μL) was added to wells (The final concentration: 100 μmol/L).
4. To mix Ammonium iron(II) sulfate and serum-free medium, the entire medium was pipetted up from wells and then immediately pipetted back one time.
5. The cells were incubated for 20 min in a 37oC incubator equilibrated with 95% air and 5% CO2, and the cells were washed with HBSS (1 mL) three times.
6. After trypsinization (250 µL), stop the reaction with serum medium (1 mL), 1.25 ml of the cell suspension was transferred to a microcentrifuge tube.
7. The cells suspension was centrifuged at 1,500 rpm for 3 minutes.
8. The supernatant was discarded and HBSS (1 mL) was added to the microcentrifuge tube and suspended by pipetting.
9. The cells suspension was centrifuged at 1,500 rpm for 3 minutes and the supernatant was discarded.
10. 1 μmol/L FerroOrange in MEM (serum-free medium) (300 μL) was added to the cells.
11. The cells were incubated for 15 -30 min in a 37oC incubator equilibrated with 95% air and 5% CO2.
12. The stained cells were passed through a cell strainer and analyze samples using a flow cytometer.
1. FerroOrange leaks into the extracellular space due to washing cells after staining; therefore, we suggest you measure cells immediately after staining (Non wash).
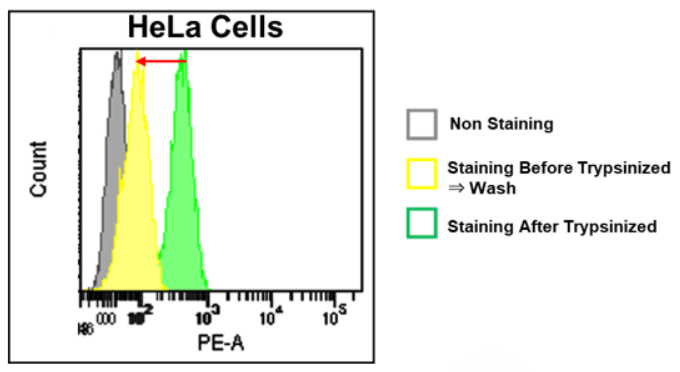
2. We recommend you add equal volumes of dye solutions, because fluorescence intensity can sometimes vary in a dye solution volume-dependent manner.
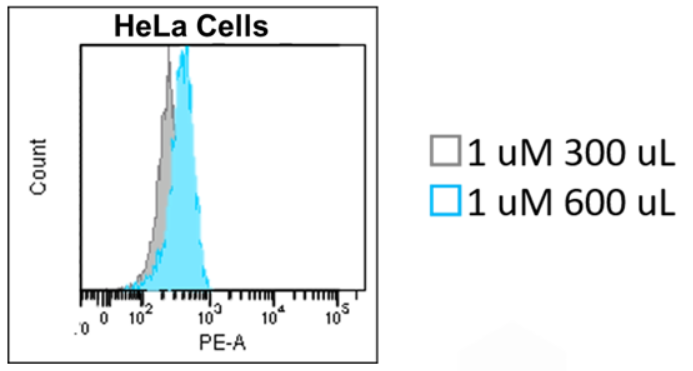
3. To verify experimental conditions for detecting Fe(II), we recommend you prepare a sample containing ammonium iron (II) sulfate and then observe changes in the fluorescence intensity of FerroOrange.
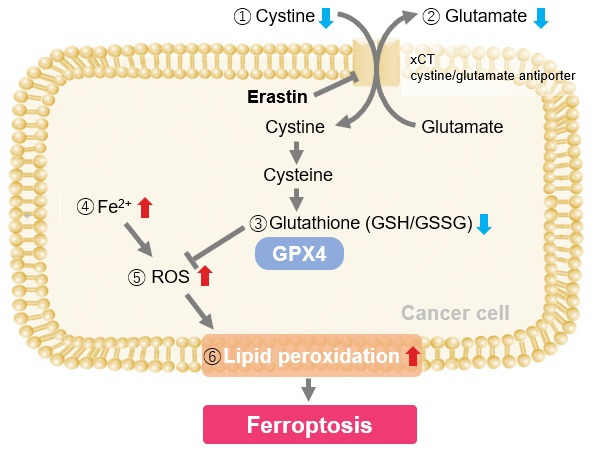
Induction of Ferroptosis by Erastin
Erastin is a known inducer of ferroptosis. By inhibiting the cystine transporter (xCT), erastin inhibits the uptake of cystine. Cystine is the raw material for GSH. Therefore, Erastin ultimately decreases the amount of GSH. Decreased GSH then results in lipid peroxide accumulation and induction of ferroptosis.
The following experimental examples show changes in each aforementioned index as a consequence of erastin stimulation. Measurements are made using Dojindo reagents.
Using erastin-treated A549 cells, we measured intracellular Fe2+, ROS, lipid peroxide, glutathione, glutamate release into the extracellular space, and cystine uptake. As a result, inhibition of xCT by elastin was observed and also the release of glutamate and uptake of cystine were decreased. Furthermore, elastin treatment decreased intracellular glutathione while it increased intracellular Fe2+ , ROS, and lipid peroxides.

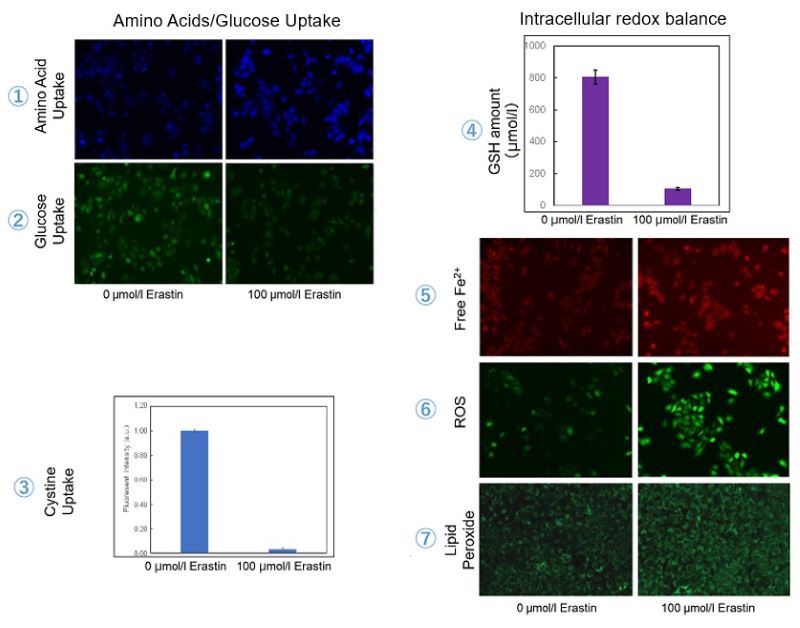
| 1 Amino Acid Uptake | : Amino Acid Uptake Assay Kit (Code: UP04) |
| 2 Glucose Uptake | : Glucose Uptake Assay Kit-Green (Code: UP02) |
| 3 Cystine Uptake | : Cystine Uptake Assay Kit (Code: UP05) |
| 4 Intracellular glutathione | : GSSG/GSH Quantification Kit (Code: G257) |
| 5 Intracellular labile Fe | : FerroOrange (Code: F374) |
| 6 Intracellular total ROS | : ROS Assay Kit -Highly Sensitive DCFH-DA- (Code: R252) |
| 7 Lipid Peroxides | : Liperfluo (Code: L248) |
Cell Line: A549
Incubation Conditions: 100 μmol/l Erastin/MEM, 37℃, 3h
References
| No. | Sample | Instruments | Publications |
|---|---|---|---|
| 1) | Cell (HeLa) |
Fluorescence | K. Tomita, M. Fukumoto, K. Itoh, Y. Kuwahara, K. Igarashi, T. Nagasawa, M. Suzuki, A. Kurimasa and T. Sato, "MiR-7-5p is a key factor that controls radioresistance via intracellular Fe2+ content in clinically relevant radioresistant cells.", Biochem Biophys Res Commun.., 2019,doi: 10.1016/j.bbrc.2019.08.117. |
| 2) | Cell (Human endothelial cell line) |
Fluorescence | Y. Wang and M. Tang, "PM2.5 induces ferroptosis in human endothelial cells through iron overload and redox imbalance", Environ. Pollut., 2019, 264, doi: 10.1016/j.envpol.2019.07.105. |
| 3) | Cell (MCF7-ADR) |
Fluorescence | S Guo, X Yao, Q Jiang, K Wang, Y Zhang, H. Peng, J. Tang and W. Yang , "Dihydroartemisinin-Loaded Magnetic Nanoparticles for Enhanced Chemodynamic Therapy", Front Pharmacol , 2020, 11, 226. |
| 4) | Cell (293T) |
Fluorescence microscope |
R. A. Weber, F. S. Yen, S.P.V. Nicholson, H. Alwaaseem, E.C. Bayraktar,M. Alam, R. C. Timson, K. La, M. Abu-Remaileh, H. Molina and K. Birsoy, "Maintaining Iron Homeostasis Is the Key Role of Lysosomal Acidity for Cell Proliferation", Mol. Cell, 2020, 77, 1-11. |
| 5) | Cell (SK-HEP-1) |
Fluorescence | X. Li, T. Wang, X. Huang, Y. Li, T. Sun, S. Zang, K. Guan, Y. Xiong, J. Liu and H. Yuan , "Targeting ferroptosis alleviates methionine‐choline deficient (MCD)‐diet induced NASH by suppressing liver lipotoxicity", Liver Int., 2020, doi:10.1111/liv.14428. |
| 6) | Cell (HepG2) |
Fluorescence |
T. Hirayama, M. Niwa, S. Hirosawa and H. Nagasawa, "High-Throughput Screening for the Discovery of Iron Homeostasis Modulators Using an Extremely Sensitive Fluorescent Probe", ACS Sens., 2020,doi: 10.1021/acssensors.0c01445. |
Q & A
-
Q
Are there any tips for successful assay?
-
A
1. Please do not change the media after adding the FerroOrange. By changing the media, the FerroOrange dye can leak out of cells.
2. For data reliability, we recommend preparing Bpy (2,2‘-bipyridine) or ammonium iron sulfate (II) treated cells as the control for comparison with FerroOrange data.
3. If the cell samples have difficulty in staining, please increase the concentration of FerroOrange working solution 1 μmol/L higher than the recommended concentration. We recommend 1-5μmol/L.
-
Q
How can I use FerroOrange with a plate reader?
-
A
Please refer to the following experimental example:
A: No dye added (HeLa cells only)
B: Bpy (2,2‘-bipyridine) treated HeLa cells
C: Ammonium iron sulfate (II) treated HeLa cells
1. Add 100μL HeLa cell suspension to each well of 96 well plate (black with clear bottom) making the final concentration 10,000 cells/well. Incubate overnight in the 5% CO2 at 37 ℃.
2. Wash Sample C with 100 μL MEM (no FBS) media three times
3. Add 100 μL ammonium iron sulfate (II)/MEM (no FBS) solution (final concentration: 100 μmol/L) to Sample C’s wells. Incubate for 30 minutes in the 5% CO2 at 37 ℃.
4. Wash cells with 100 μL HBSS three times
5. Add 100 μL of 1 μmol/L FerroOrange working solution to Sample A and Sample C’s wells. Add 100 μL of HBSS solution containing FerroOrange (final concentration: 1 μmol/L) and Bpy (final concentration: 100 μmol/L) to Sample B’s wells. Incubate for 30 minutes in the 5% CO2 at 37 ℃.
6. Measure the fluorescent intensity (Ex. 543 nm, Em. 580nm) using a fluorescence microplate reader.
Ex/Em = 543 nm/ 580 nm
Left Control
Middle 2,2′-Bipyridyl (Bpy) treated
Right Ammonium iron (II) sulfate treated
The change of intracellular Fe2+in HeLa cells was quantified by the FerroOrange.
-
Q
Is it necessary to wash cells after staining?
-
A
We recommend you not to wash them because this step may induce leakage of FerroOrange from the cells.
It has been confirmed that the amount of FerroOrange leakage differs depending on cell density and cell type.<The effect of cell types and density on FerroOrange leakage>
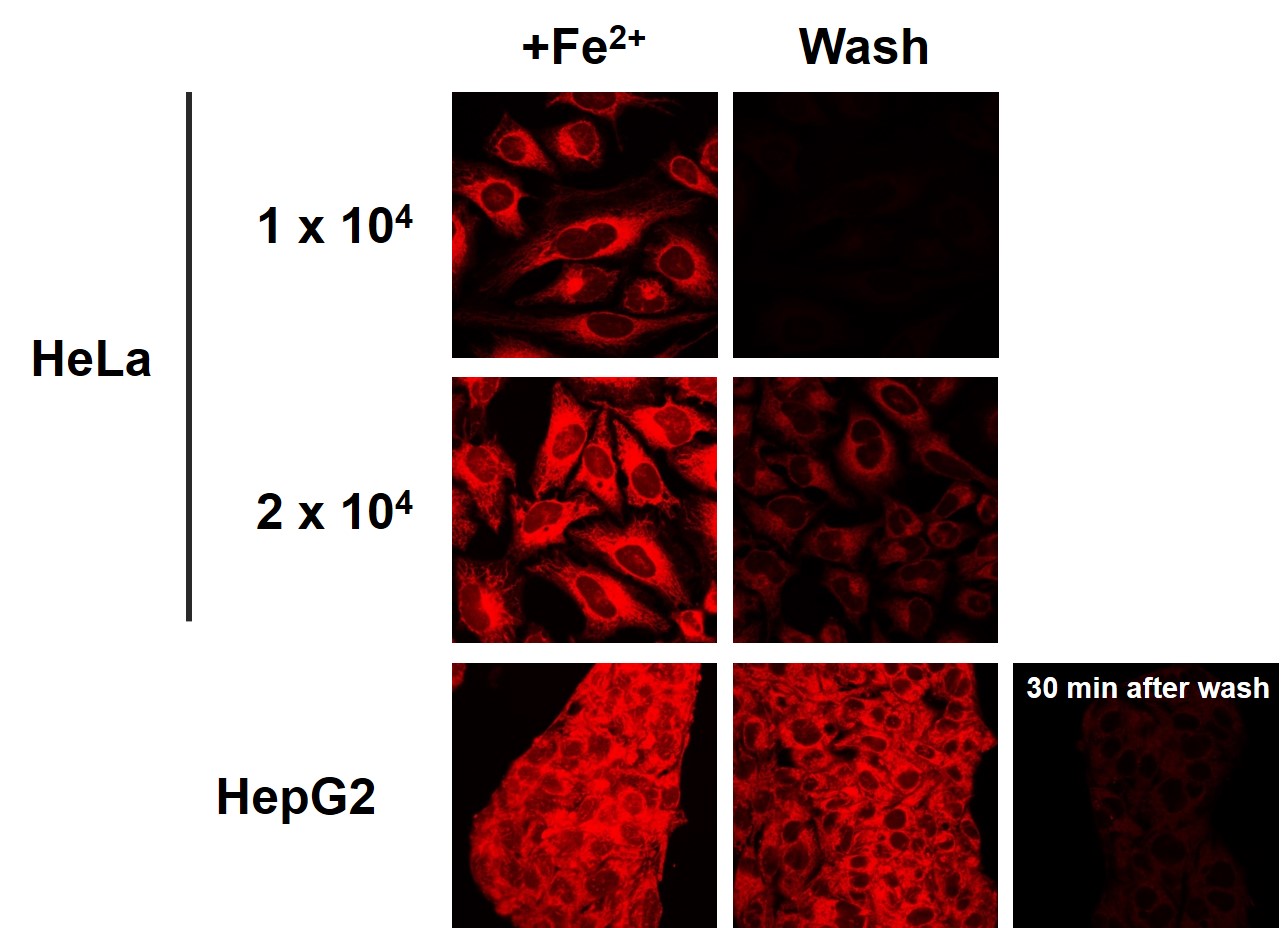
-
Q
What is the recommended filter?
-
A
Excitation: 530-565 nm; Emission: 570-620 nm
-
Q
Is this dye compatible with fixed cells?
-
A
No.
FerroOrange signal is undetected and the changes in intracellular iron(II) ions cannot be confirmed in fixed cells.
HepG2 cells were treated with ammonium sulfate iron(II) (final concentration: 100 µmol/L) for 30 minutes.
After washing, FerroOrange Working solution was added to unfixed, fixed for 15 minutes, and fixed for 30 minutes cells, and the cells were observed by fluorescence microscopy.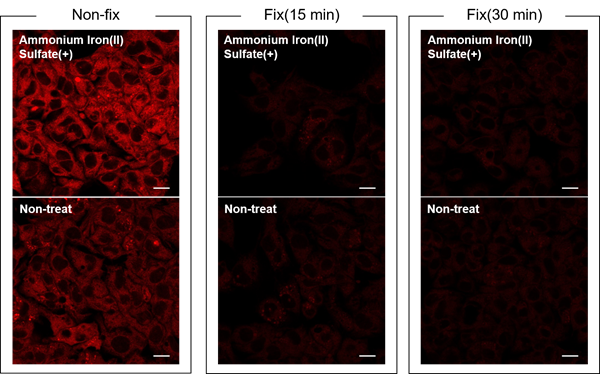
Detecting Conditions
Ex: 561 nm(1.50 %)
Em: 550-650 nm
Gain: 700 V
Scale bar: 20 µm
-
Q
Is this dye compatible with floating cells?
-
A
Yes, please refer to the following protocol.
FerroOrange working solution was added to Jurkat cells with iron(II) ammonium sulfate (final concentration: 100 µmol/l) and observed by fluorescence microscopy.
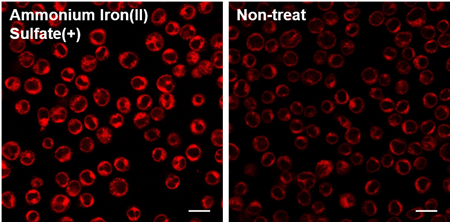
Detecting Conditions
Ex: 561 nm(1.50 %)
Em: 550-650 nm
Gain: 700 V
Scale bar: 20 µm
Protocol
1. Jurkat cell suspensions of 3×106 cells/ml (using serum-free MEM) were seeded into dishes and 10 mmol/l ammonium iron(II) sulfate solution was added. (final concentration 100 µmol/l)
2. Incubated in an incubator (37 ℃, 5 ℃, in the presence of 5 % CO2) for 30 min.
3. Cells were transferred to a 1.5 ml tube, and centrifuged.
After removing the supernatant, cells were washed once with HBSS.
4. Cells were centrifuged to remove the supernatant. 1 µmol/l FerroOrange working solution was added to cells and transferred to an ibidi 8-well plate.
5. Incubated in an incubator (37 ℃, 5 ℃, in the presence of 5 % CO2) for 30 min.
6. Observed by fluorescence microscopy
-
Q
Are there any possible causes for low S/N?
-
A
Serum may affect the result.
We have observed that the background is increased when using a serum containing medium compared to a serum free medium.FerroOrange Working solution prepared in a serum containing or serum free medium was added to A549 cells treated with 10 µM Erastin for 24 hours and then observed by fluorescence microscopy.
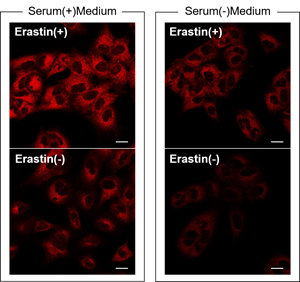
Detecting Conditions
Ex: 561 nm(1.50 %)
Em: 550-650 nm
Gain: 700 V
Scale bar: 20 µm
Handling and storage condition
| Appearance: | |
|---|---|
| Purity (HPLC): | ≧ 92.0 % |
| Fluorescence spectrum: | To pass test |
| 0-5°C, Protect from light |