General Information
Iron is the most abundant transition metal element within organisms, and it participates in various physiological activities. Recently, free iron in living cells has attracted attention because its high reactivity may be related to cellular damage or death. Free iron exists in its stable redox states, namely ferrous ion (Fe2+) and ferric ion (Fe3+). In living cells, understanding the behavior of Fe2+ is considered more important than understanding that of Fe3+ because of the intracellular reductive environment, metal transporters, and the water solubility of Fe2+. FerroOrange is a novel fluorescent probe that enables live-cell fluorescent imaging of intracellular Fe2+.
Figure 1. Detection of intracellular Fe2+ using FerroOrange
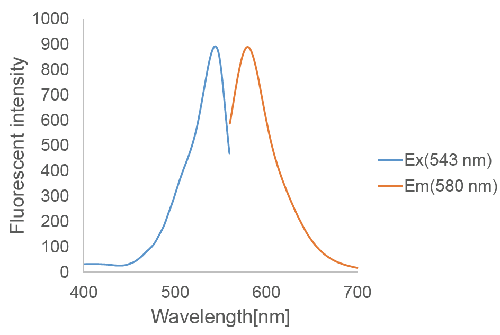
Figure 2. Excitation and emission spectra of FerroOrange
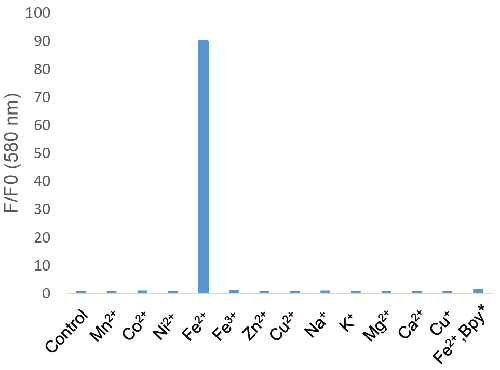
*Bpy; 2,2'-Bipyridyl
Figure 3. Metal ion selectivity of FerroOrange
Contents
| 1 tube | 3 tubes | |
| FerroOrange | 24 μg x 1 | 24 μg x 3 |
Storage Condition
Store at 0–5 ℃ and protect from light.
Required Equipment and Materials
- Dimethyl sulfoxide (DMSO), Dimethylformamide (DMF), or Ethanol
- HBSS
- Serum-free medium
- Micropipettes
Preparation of the Solution
Preparation of FerroOrange working solution
Add 35 μl of DMSO to a tube containing 24 μg of FerroOrange and dissolve the solution via pipetting to prepare a 1 mmol/l FerroOrange solution. Dilute the 1mmol/l FerroOrange solution with HBSS to prepare the 1 μmol/l FerroOrange working solution.
- DMF or ethanol can also be used alternatively to DMSO for preparation. To protect from light, keep this solution in the aluminium bag and store at -20℃. The 1 mmol/l FerroOrange solution is stable for 1 month under this condition. The 1 μmol/l FerroOrange working solution is unstable. Please prepare the solutions immediately before staining the experiment and use them immediately.
- Substitute serum-free medium for HBSS if needed. However, please note that serum-containing medium cannot be used because it causes high background.
General Protocol
- 1. Seed cells on a dish for fluorescent imaging and culture them overnight in a 37℃ incubator equilibrated with 95% air and 5% CO2.
- 2. Discard the supernatant and wash the cells with HBSS or serum-free medium three times.
- 3. Add medium containing stimulating agents and incubate the cells in a 37℃ incubator equilibrated with 95% air and 5% CO2.
- Please optimize the incubation time according to the stimulation conditions.
- 4. Discard the supernatant and wash the cells with HBSS or serum-free medium three times.
- 5. Add the 1 μmol/l FerroOrange working solution to the cells and incubate them for 30 min a 37℃ incubator equilibrated with 95% air and 5% CO2.
- 6. Observe the cells under a fluorescence microscope.
- Do not wash the cells after step 5.
Experimental Example 1
Detection of intracellular Fe2+ in HeLa cells using FerroOrange.
- HeLa cells (2.0×104 cells/well) were seeded on a μ-slide 8 well (ibidi) and cultured overnight in a 37℃ incubator equilibrated with 95% air and 5% CO2.
- The cells were washed with serum-free medium (200 μl) three times. Then, serum-free medium (200 μl) was added to the cells.
- Ammonium iron (II) sulfate (10 mmol/l) was prepared with purified water.
- Ammonium iron (II) sulfate (2 μl) was added to wells (The final concentration:100 μmol/l). To mix Ammonium iron (II) sulfate and serum-free medium, the entire medium was pipetted up from wells and then immediately pipetted back one time.
- Please do not disturb the cells during pipetting.
- When adding 10 mmol/l Ammonium iron (II) sulfate to well, please exactly follow step 4 as described. Do not add pre-prepared 100 μmol/l Ammonium iron (II) sulfate to cells. It may result in precipitation of Ammonium iron (II) sulfate during the experiment due to a vortex or a pipetting.
- The cells were incubated for 30 min in a 37℃ incubator equilibrated with 95% air and 5% CO2, and the cells were washed with HBSS (200 μl) three times.
- FerroOrange (1 μmol/l) and 2,2'-bipyridyl (Bpy) (100 μmol/l) were added to the cells as HBSS solution (200 μl), and then cells were incubated for 30 min in a 37℃ incubator equilibrated with 95% air and 5% CO2.
- The cells were observed under a confocal fluorescence microscope.
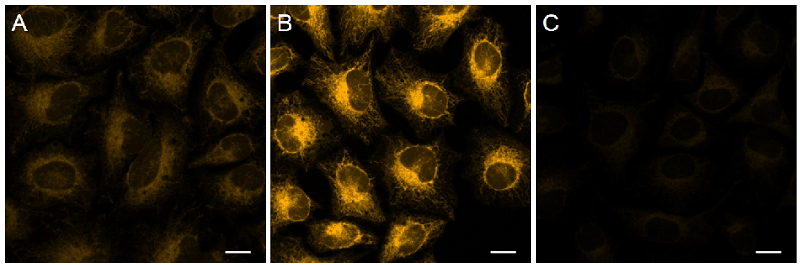
Figure 4. Detection of intracellular Fe2+ in HeLa cellsin cells using FerroOrange.
| Ex/Em = 561 nm/ 570-620 nm A Control B Ammonium iron(II) sulfate treated C Ammonium iron(II) sulfate and 2,2'-Bipyridyl (Bpy) treated Scale bars 20 μm |
The fluorescence intensity of FerroOrange was increased in HeLa cells treated with Ammonium iron(II) sulfate compared with the findings in untreated cells; conversely, its fluorescence intensity was decreased in cells treated with Bpy. Therefore, FerroOrange reacted with intracellular Fe2+.
Experimental Example 2
Detection of intracellular Fe2+ in HeLa cells using FerroOrange.
- HeLa cells (2.0×104 cells/well) were seeded on a μ-slide 8 well (ibidi) and cultured overnight in a 37℃ incubator equilibrated with 95% air and 5% CO2.
- The cells were washed with HBSS (200 μl) three times.
- FerroOrange (1 μmol/l) and Bpy (100 μmol/l) were added to the cells as HBSS solution (200 μl), and the cells were incubated for 30 min in a 37℃ incubator equilibrated with 95% air and 5% CO2.
- The cells were observed under a confocal fluorescence microscope.
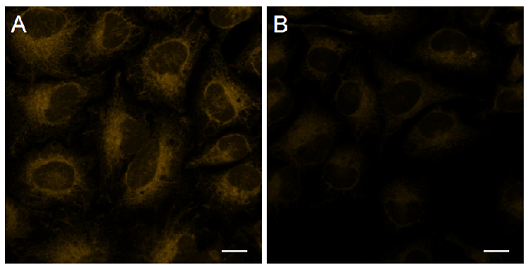
Figure 5. Detection of intracellular Fe2+ in HeLa cells using FerroOrange.
| Ex/Em = 561 nm/ 570-620 nm A Control B 2,2'-Bipyridyl (Bpy) treated Scale bars 20 μm |
The fluorescence intensity of FerroOrange was decreased in HeLa cells treated with Bpy compared with that in untreated cells. Therefore, FerroOrange reacted with intracellular Fe2+.
This product was commercialized under the advisory of Dr. Hideko Nagasawa and Dr. Tasuku Hirayama (Gifu Pharmaceutical University).
Frequently Asked Questions / Reference
F374: FerroOrange
Revised Aug., 22, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.