|
Recent research shows that monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) play distinct roles in lipid metabolism and ferroptosis, a form of regulated cell death driven by lipid peroxidation. Here are some of the papers that highlight the mechanisms and factors by which MUFAs and PUFAs modulate ferroptosis for cellular homeostasis and disease implications.
Monounsaturated fatty acids (MUFAs) and polyunsaturated fatty acids (PUFAs) play distinct roles in lipid metabolism and ferroptosis, a form of regulated cell death driven by lipid peroxidation. PUFAs, with their multiple double bonds, are highly susceptible to peroxidation, making them key drivers of ferroptosis. In contrast, MUFAs, with their single double bond, are less susceptible to peroxidation and may counteract ferroptosis by reducing the availability of oxidizable lipids. This dynamic highlights the opposing roles of MUFAs and PUFAs in modulating ferroptosis, which has implications for cancer therapy and other diseases involving oxidative stress.
|
-
Breast cancer secretes anti-ferroptotic MUFAs and depends on selenoprotein synthesis for metastasis
Click here for the original article: Tobias Ackermann, et. al., EMBO Mol. Med., 2024.
Point of Interest
- Ferroptosis, driven by iron-dependent lipid peroxidation, is counteracted by GPX4 and inhibited by monounsaturated fatty acid (MUFA) secretion and stearoyl-CoA desaturase (SCD) expression.
- Low SCD expression at low cell density increases the susceptibility of triple-negative breast cancer (TNBC) cells to ferroptosis, which is associated with reduced anti-ferroptotic MUFA secretion.
- Inhibition of Sec-tRNAsec biosynthesis, an essential step for selenoprotein production, induces ferroptosis, which impairs TNBC cell metastasis by disrupting selenoprotein production and MUFA protection.
-
Phospholipids with two polyunsaturated fatty acyl tails promote ferroptosis
Click here for the original article: Baiyu Qiu et. al., Cell, 2024.
Point of Interest
- Diacyl polyunsaturated fatty acid phosphatidylcholines (PC-PUFA2s) drive ferroptosis by generating mitochondrial ROS, initiating lipid peroxidation and influencing cancer cell sensitivity.
- Depletion of PC-PUFA2s in aging and disease is associated with ferroptosis, with mitochondrial antioxidants providing protection.
- PC-PUFA2s regulate mitochondrial homeostasis and ferroptosis, providing potential diagnostic and therapeutic targets for ferroptosis modulation.
-
Polyunsaturated fatty acids-induced ferroptosis suppresses pancreatic cancer growth
Click here for the original article: Akane Suda et. al., Cancer Discovery, 2024.
Point of Interest
- Linoleic acid (LA) and α-linolenic acid (αLA), one of the PUFA, induce ferroptosis in pancreatic cancer cells, reducing tumour growth and improving survival in mice.
- PUFA-induced ferroptosis varies by type; Arachidonic acid and Eicosapentaenoic acid (EPA) show strong effects on pancreatic cancer, while Docosahexaenoic acid mildly suppresses proliferation.
- EPA induced ferroptosis in colorectal adenocarcinoma, while LA and αLA did not show similar effects in this context.
- PUFAs show potential as anti-cancer agents via ferroptosis, effective even in gemcitabine-resistant pancreatic cancer cells.
|
|
Related Techniques
|
- Intracellular / mitochondrial ferrous ion (Fe2+) detection
- FerroOrange(intracellular), Mito-FerroGreen(mitochondrial)
|
- Cellular senescence detection
- SPiDER-βGal for live-cell imaging or flow cytometry / microplate reader / tissue samples
SPiDER-βGal Blue for fixed cell and for multiple staining with immunostaining and other methods
|
|
|
- Lipid Peroxidation Assay
- Lipid Peroxidation Probe -BDP 581/591 C11-
|
- Total ROS detection
- Highly sensitive DCFH-DA or Photo-oxidation Resistant DCFH-DA
|
- Mitochondrial superoxide detection
- MitoBright ROS Deep Red - Mitochondrial Superoxide Detection
|
- Glutathione Quantification
- GSSG/GSH Quantification Kit
|
- Cystine Uptake detection
- Cystine Uptake Assay Kit
|
- MDA detection
- MDA Assay Kit
|
- Glycolysis/Oxidative phosphorylation Assay
- Glycolysis/OXPHOS Assay Kit
|
- Apoptosis detection in multiple samples
- Annexin V Apoptosis Plate Assay Kit
|
- Cell proliferation/ cytotoxicity assay
- Cell Counting Kit-8 and Cytotoxicity LDH Assay Kit-WST
|
|
Related Applications
|
Erastin-Induced Ferroptosis: Evaluating Intracellular Uptake and Redox Balance
We investigated the transition of cellular metabolisms in A549 cells treated with erastin, a known ferroptosis inducer. Our results revealed the following.
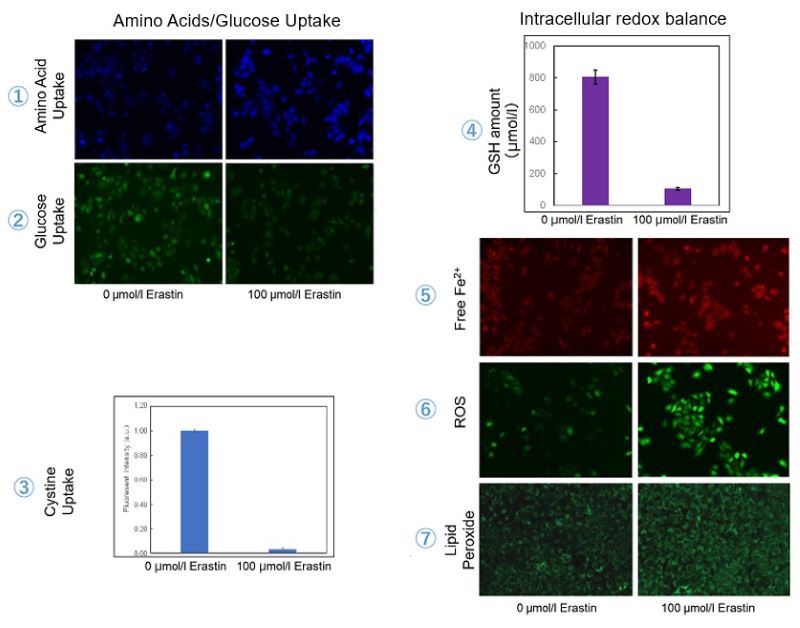
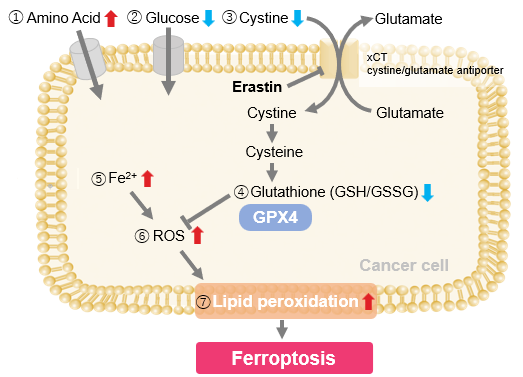
- The inhibition of cystine uptake by erastin led to a depletion of cysteine, which in turn increased the compensatory uptake of other amino acids.
- Glucose uptake, which typically promotes ferroptosis*, was found to decrease upon erastin treatment, suggesting a potential cellular self-defense mechanism.
- The depletion of cysteine resulted in a decrease in glutathione levels and an increase in Fe2+, ROS, and lipid peroxides, all of which are recognized markers of ferroptosis.
Cell Line: A549
Incubation Conditions: 100 μmol/l Erastin/MEM, 37℃, 3h
*Reference: Xinxin Song, et al., Cell Reports, (2021)
Products in Use
|

















