|
In recent cancer research, ferroptosis, a type of regulated cell death involving iron-dependent lipid peroxidation, has shown promise in cancer treatment. Here are some of the papers that have identified mechanisms of ferroptosis resistance in cancer cells and suggested that they may be therapeutic targets.
Ferroptosis, a type of regulated cell death involving iron-dependent lipid peroxidation, has shown promise in cancer treatment. It provides an alternative pathway to eliminate cancer cells that are resistant to traditional therapies such as apoptosis. By targeting ferroptosis, researchers can exploit metabolic weaknesses in cancer cells, particularly when antioxidant defenses are disrupted. This approach is being explored to improve the efficacy of existing treatments and potentially overcome drug resistance in several types of cancer.
|
-
7-Dehydrocholesterol is an endogenous suppressor of ferroptosis
Click here for the original article: Florencio Porto Freitas, et. al., Nature, 2024.
Point of Interest
- High concentrations of 7-DHC are cytotoxic to developing neurons by promoting lipid peroxidation.
- On the other hand, 7-DHC accumulation confers a prosurvival function in cancer cells.
- 7-DHC effectively protects (phospho)lipids from autoxidation and subsequent fragmentation due to its superior reactivity with peroxyl radicals.
- Accumulation of 7-DHC induces a shift to a ferroptosis-resistant state in tumors.
-
Tumor suppressor Par-4 activates autophagy-dependent ferroptosis
Click here for the original article: Karthikeyan Subburayan et. al., Communications Biology, 2024.
Point of Interest
- Point o- Par-4/PAWR activation is critical during ferroptosis, with its depletion blocking and overexpression enhancing this ferroptosis process.
- Upregulation of Par-4 promotes NCOA4-dependent ferritinophagy, a selective autophagy process that degrades ferritin, increasing free iron release, lipid peroxidation, and ROS production.
- Par-4 inhibition blocks ferroptosis-driven tumor suppression, highlighting its potential for cancer therapy.
-
BRCA1-Mediated Dual Regulation of Ferroptosis Exposes a Vulnerability to GPX4 and PARP Co-Inhibition in BRCA1-Deficient Cancers
Click here for the original article: Jonathan Sun et. al., Cancer Discovery, 2024.
Point of Interest
- BRCA1 deficiency in cancers confers resistance to erastin-induced ferroptosis but increases susceptibility to GPX4 inhibitor-induced ferroptosis.
- Combined PARP and GPX4 inhibition in BRCA1-deficient cancers promotes ferroptosis, providing a strategy to overcome PARP inhibitor resistance.
- Reduced GPX4 expression and NCOA4-induced ferritinophagy in BRCA1-mutant cancers increases sensitivity to co-treatment with PARP and GPX4 inhibitors.
|
|
Related Techniques
|
- Intracellular / mitochondrial ferrous ion (Fe2+) detection
- FerroOrange(intracellular), Mito-FerroGreen(mitochondrial)
|
- Cellular senescence detection
- SPiDER-βGal for live-cell imaging or flow cytometry / microplate reader / tissue samples
SPiDER-βGal Blue for fixed cell and for multiple staining with immunostaining and other methods
|
|
|
- Lipid Peroxidation Assay
- Lipid Peroxidation Probe -BDP 581/591 C11-
|
- Total ROS detection
- Highly sensitive DCFH-DA or Photo-oxidation Resistant DCFH-DA
|
- Mitochondrial superoxide detection
- MitoBright ROS Deep Red - Mitochondrial Superoxide Detection
|
- Glutathione Quantification
- GSSG/GSH Quantification Kit
|
- Cystine Uptake detection
- Cystine Uptake Assay Kit
|
- MDA detection
- MDA Assay Kit
|
- First-time autophagy research
- Autophagic Flux Assay Kit
|
- Glycolysis/Oxidative phosphorylation Assay
- Glycolysis/OXPHOS Assay Kit
|
- Apoptosis detection in multiple samples
- Annexin V Apoptosis Plate Assay Kit
|
- Mitochondrial membrane potential detection
- JC-1 MitoMP Detection Kit, MT-1 MitoMP Detection Kit
|
- Cell proliferation/ cytotoxicity assay
- Cell Counting Kit-8 and Cytotoxicity LDH Assay Kit-WST
|
|
Related Applications
|
Erastin-Induced Ferroptosis: Evaluating Intracellular Uptake and Redox Balance
We investigated the transition of cellular metabolisms in A549 cells treated with erastin, a known ferroptosis inducer. Our results revealed the following.
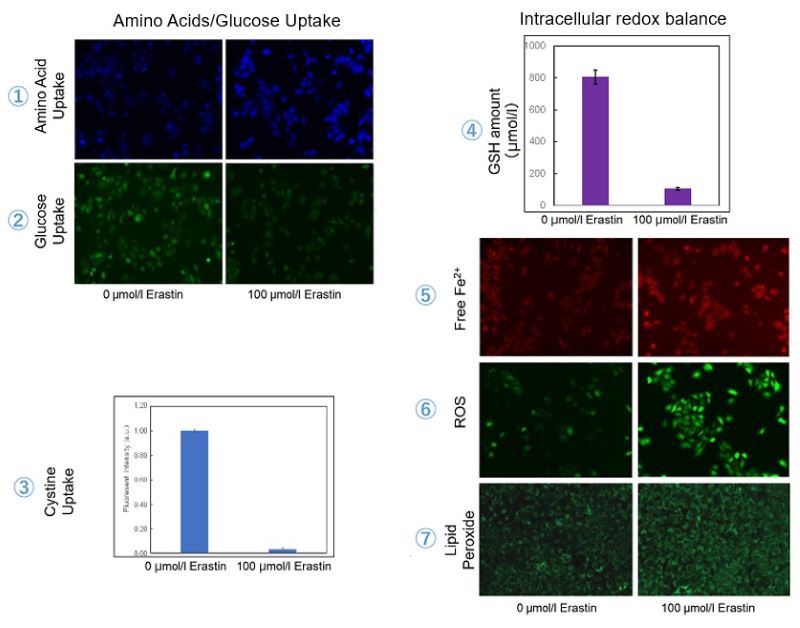
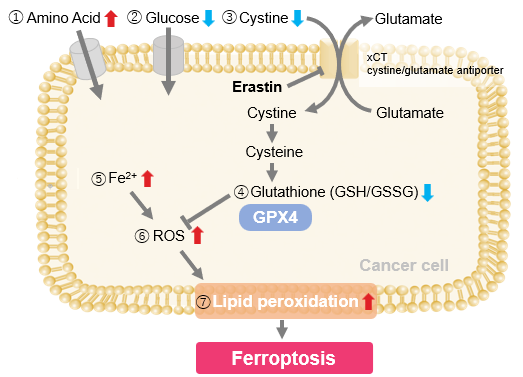
- The inhibition of cystine uptake by erastin led to a depletion of cysteine, which in turn increased the compensatory uptake of other amino acids.
- Glucose uptake, which typically promotes ferroptosis*, was found to decrease upon erastin treatment, suggesting a potential cellular self-defense mechanism.
- The depletion of cysteine resulted in a decrease in glutathione levels and an increase in Fe2+, ROS, and lipid peroxides, all of which are recognized markers of ferroptosis.
Cell Line: A549
Incubation Conditions: 100 μmol/l Erastin/MEM, 37℃, 3h
*Reference: Xinxin Song, et al., Cell Reports, (2021)
Products in Use
|
| ① Amino Acid Uptake :Amino Acid Uptake Assay Kit |
| ② Glucose Uptake: Glucose Uptake Assay Kit-Green |
| ③ Cystine Uptake: Cystine Uptake Assay Kit |
| ④ Intracellular glutathione: GSSG/GSH Quantification Kit |
| ⑤ Intracellular labile Fe : FerroOrange |
| ⑥ Intracellular total ROS: ROS Assay Kit -Highly Sensitive DCFH-DA- |
| ⑦ Lipid Peroxides : Liperfluo |

















