|
Mitochondrial metabolism involves the biochemical processes within mitochondria that convert nutrients into energy and building blocks necessary for cell function, primarily through the citric acid cycle and oxidative phosphorylation. This energy production produces adenosine triphosphate (ATP), the cell's primary energy currency. Oxidative stress occurs when there's an imbalance between the production of reactive oxygen species (ROS) in the mitochondria and the cell's ability to detoxify these harmful byproducts or repair the resulting damage. Over time, excessive oxidative stress can lead to cellular damage that contributes to aging and several diseases, including neurodegenerative disorders and cancer.
|
-
Autoregulatory control of mitochondrial glutathione homeostasis
Click here for the original article: Yuyang Liu, et. al., Science, 2023.
Point of Interest
- The mitochondrial glutathione (GSH) transporter, SLC25A39, undergoes rapid degradation by the mitochondrial protease AFG3L2.
- Depletion of GSH dissociates AFG3L2 from SLC25A39, which triggers enhancement in the uptake of GSH by mitochondria.
- This regulatory mechanism is dependent on a putative iron-sulfur cluster within SLC25A39, linking the control of mitochondrial iron homeostasis to GSH import.
-
ApoE enhances mitochondrial metabolism via microRNA-142a/146a-regulated circuits that suppress hematopoiesis and inflammation in hyperlipidemia
Click here for the original article: Tuan Anh Phu et. al., Cell Reports, 2023.
Point of Interest
- Apolipoprotein E (ApoE) elevates the levels of miR-146a in myeloid cells and hematopoietic stem and progenitor cells, leading to decreased glucose absorption and glycolysis.
- By diminishing miR-142a levels, ApoE enhances fatty acid oxidation, thereby improving mitochondrial metabolism.
- ApoE plays a critical role in regulating immune cell metabolism, influencing hematopoiesis and inflammation in conditions of hyperlipidemia.
-
Mitochondrial protein C15ORF48 is a stress-independent inducer of autophagy that regulates oxidative stress and autoimmunity
Click here for the original article: Yuki Takakura et. al., Nature Communications, 2024.
Point of Interest
- The mitochondrial protein C15ORF48 reduces mitochondrial membrane potential and intracellular ATP levels, resulting in the activation of Unc-51-like kinase 1.
- C15ORF48-dependent induction of autophagy upregulates intracellular glutathione levels and promotes cell survival by reducing oxidative stress.
- Mice deficient in C15orf48 show a reduction in stress-independent autophagy in thymic epithelial cells (TECs).
- C15orf48-/- mice develop autoimmunity, suggesting that stress-independent autophagy in TECs is critical for thymic self-tolerance.
|
| Related Techniques |
|
|
|
|
|
|
|
|
|
|
|
|
- Mitochondrial staining (Long-Term Visualization)
- MitoBright LT Green, Red, Deep Red
|
|
|
|
|
| Related Applications |
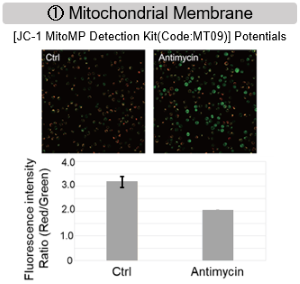
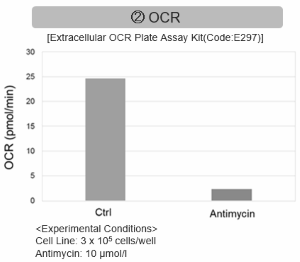
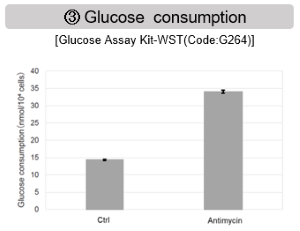
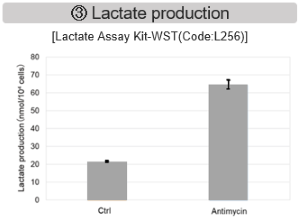
Inhibition of Mitochondrial Electron Transport Chain
-
-
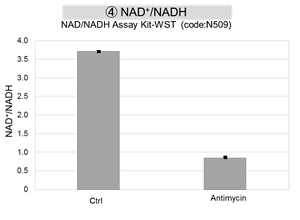
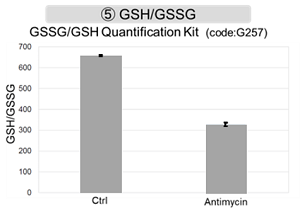
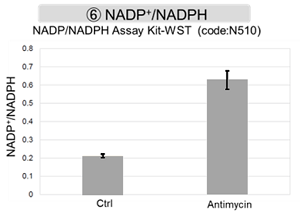
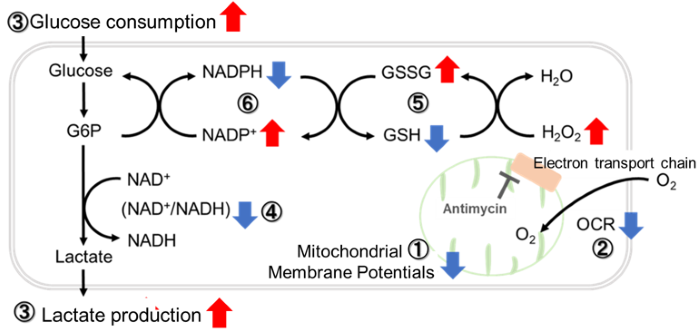
Antimycin stimulation of Jurkat cells was used to evaluate the changes in cellular state upon inhibition of the mitochondrial electron transport chain using a variety of indicators.
The results showed that inhibition of the electron transport chain resulted in (1) a decrease in mitochondrial membrane potential and (2) a decrease in OCR. In addition, (3) the NAD+/NADH ratio of the entire glycolytic pathway decreased due to increased metabolism of pyruvate to lactate to maintain the glycolytic pathway, (4) GSH depletion due to increased reactive oxygen species (ROS), and (6) increase in the NADP+/NADPH ratio due to decreased NADH required for glutathione biosynthesis were observed.
|
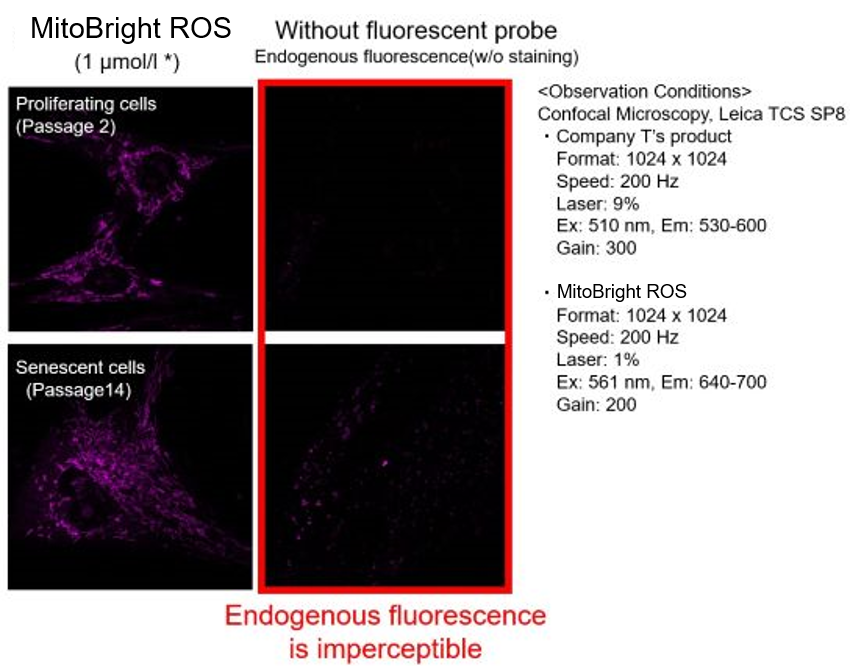
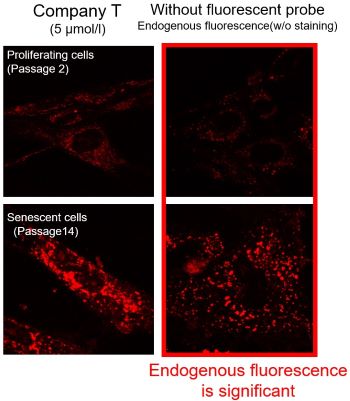
Mitochondrial Superoxide Detection in Senescent Cells
Background fluorescence caused by lipofuscin can be minimized by using a better fluorescent probe, as tested in TIG-1 cells.
Lipofuscin accumulates in senescent cells, causing increased background fluorescence during observation. To minimize the effects of endogenous fluorescence from lipofuscin and other substances, a better fluorescent probe was tested in TIG-1 cells. Company T's product exhibited endogenous fluorescence, while MitoBright ROS Deep Red showed less background fluorescence. Researchers should compare sensitivity, wavelength, and channels and select the appropriate fluorescent probe to minimize endogenous fluorescence for accurate cellular senescence research.
Products in Use
- MitoBright ROS - Mitochondrial Superoxide Detection
|