GSSG/GSH Quantification Kit - Oxidized/Reduced Glutathione Assay
Glutathione Quantification
- Optimized Enzymatic recycling system
- High reproducibility for detecting GSH, GSSG and total glutathione individually
- Contain optimized Masking Reagent for exclusive measurement of GSSG
- Easy and Simple procedure; takes 1-2 hours
-
Product codeG257 GSSG/GSH Quantification Kit - Oxidized/Reduced Glutathione Assay
| Unit size | Price | Item Code |
|---|---|---|
| 200 tests | Find your distributors | G257-10 |
| 200 tests | ・ Enzyme Solution ・ Coenzyme ・ Buffer Solution ・ Substrate (DTNB) ・ Standard GSH ・ Standard GSS |
50 μl x1 x2 60 ml x1 x4 x1 x1 20 μl x1 |
|---|
Description
Glutathione (γ-L-glutamyl-L-cysteinylglycine) is a tripeptide present in the body, and it is involved in antioxidation, drug metabolism, and other as enzyme substrate of glutathione peroxidase, glutathione S-transferase, and thiol transferase, etc. Glutathione is usually present as reduced form (GSH), but GSH is converted into its oxidized form (GSSG) by stimulation such as oxidative stress. Therefore, the ratio of GSH and GSSG has been noted as index of oxidative stress.
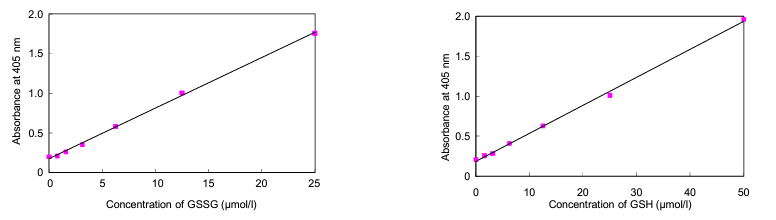
The GSSG/GSH Quantification kit contains Masking Reagent of GSH. The GSH can be deactivated in the sample by simply adding the Masking Reagent. Therefore, temp-only the GSSG is detected by measuring the absorption (λmax = 412 nm) of DTNB (5,5 Edithiobis (2-nitrobenzoic acid) using the enzymatic recycling system. Also, GSH can be determined the quantity by subtracting GSSG from the total amount of glutathione.
The kit is limited to quantifying GSH/GSSG concentration from 0.5 μmol/l to 50 μmol/l and GSSG concentration from 0.5 μmol/l to 25 μmol/l.
Ferroptosis Analysis Products
| Product Name | Target | Ditection Properties |
|---|---|---|
| FerroOrange | Intracellular Fe2+ | Microscopy, Plate reader Ex: 543 nm / Em: 580 nm |
| Mito-FerroGreen | Mitochondrial Fe2+ | Microscopy Ex: 505 nm / Em: 535 nm |
| Liperfluo | Lipid Peroxide | Microscopy, FCM Ex: 488 nm / Em: 500-550 nm |
| Lipid Peroxidation Probe -BDP 581/591 C11- |
Lipid Peroxidation Process |
Microscopy, FCM, Plate reader |
| MDA Assay Kit | Malondialdehyde | Plate reader Fluorescence, Ex: 540 nm / Em: 590 nm Colorimetric, λ: 532 nm |
| Cystine Uptake Assay Kit | Cystine uptake | Plate reader Ex: 485 nm / Em: 535 nm |
| GSSG/GSH Quantification Kit | GSSG and GSH | Plate reader Colorimetric, λ: 405 nm |
| Developer | Dojindo Molecular Technologies, Inc. |
|---|
Manual
Technical info
Principle
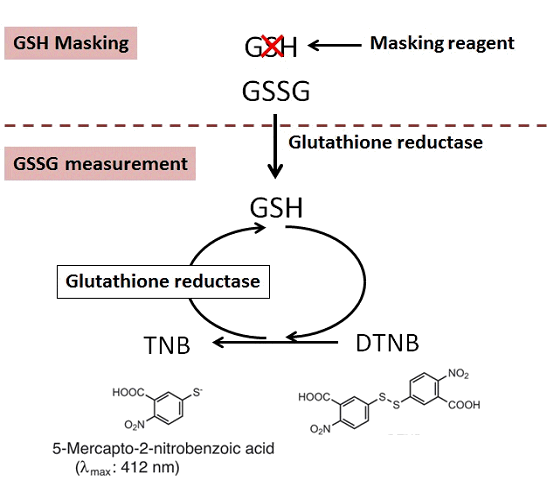
Selective Quantification
Although conventional masking reagent, 2-Vinylpyridine(2-VP), interferes the reaction of GSSG measurement, Dojindo’s masking reagent does not interfere the reaction of GSSG measurement. Therefore, the exact ratio of GSSG and GSH is obtained with Dojindo GSSG/GSH detection Kit.
Measurement of GSSG with or without GSH masking reagent
Interference
Reducing agents such as ascorbic acid, β-mercaptoethanol, dithiothreitol (DTT) and cysteine, or thiol reactive compounds such as maleimide compounds, interfere with the glutathione assay. Therefore, SH compounds, reducing agents and SH reactive materials should be avoided during the sample preparation.
Required Equipment and Materials
– Plate reader (405 or 415 nm filter)
– 96-well microplate
– Incubator (37ºC)
– 20 µl and 200 µl pipettes, a multi channel pipette
– 5-Sulfosalicylic Acid (SSA) Solution
– Ethanol
Assay Procedure
Sulfasalazine Alters Intracellular Metabolites and Increases ROS in A549 Cells
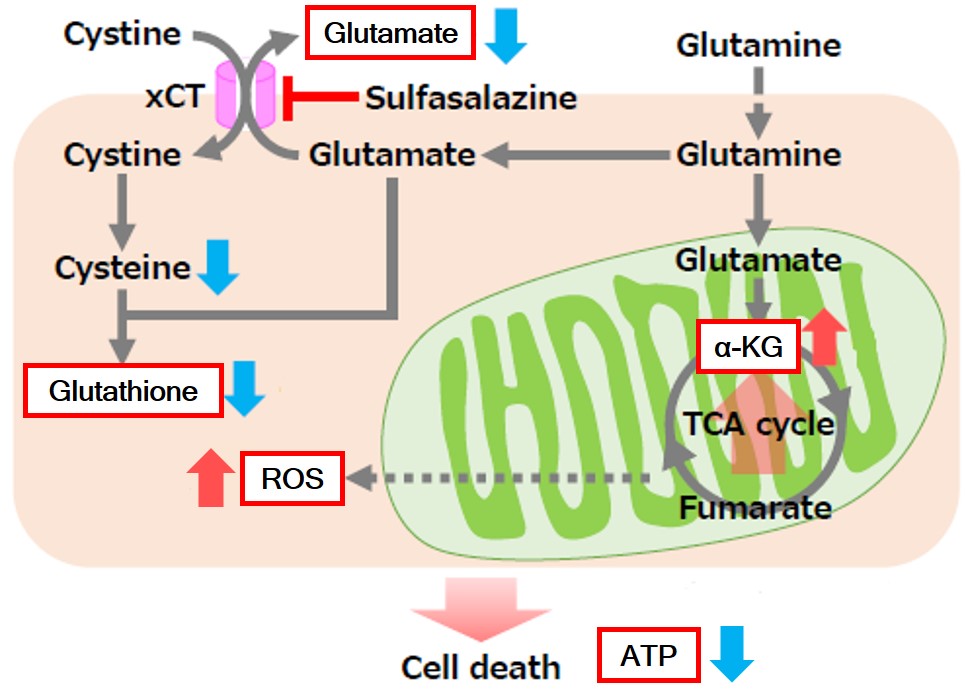 |
After addition of sulfasalazine (SSZ), a known inhibitor of cystine/glutamate transporter (xCT), to A549 cells, we observed changes in intracellular glutathione (GSH), ATP, α-ketoglutarate (α-KG), ROS and glutamate release. The results indicated that the addition of SSZ
|
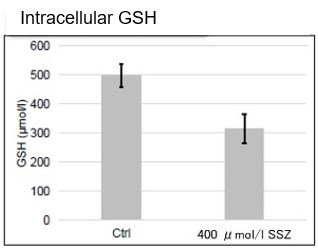 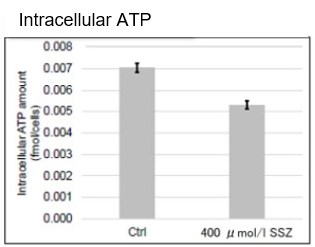 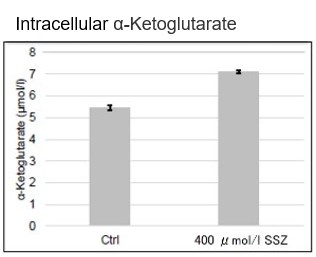 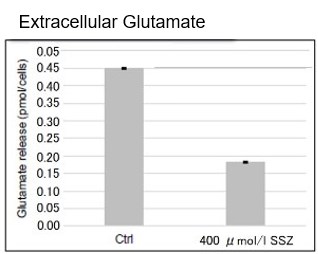 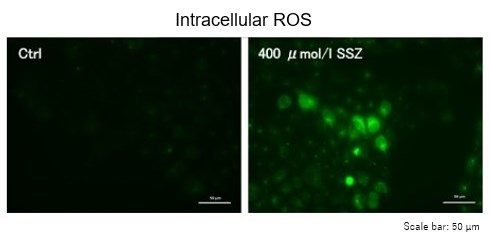 |
|
Induction of Ferroptosis by Erastin
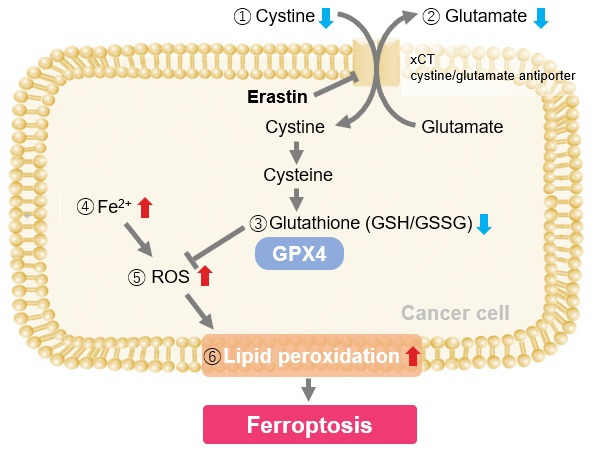
Erastin is a known inducer of ferroptosis. By inhibiting the cystine transporter (xCT), erastin inhibits the uptake of cystine. Cystine is the raw material for GSH. Therefore, Erastin ultimately decreases the amount of GSH. Decreased GSH then results in lipid peroxide accumulation and induction of ferroptosis.
The following experimental examples show changes in each aforementioned index as a consequence of erastin stimulation. Measurements are made using Dojindo reagents.
Using erastin-treated A549 cells, we measured intracellular Fe2+, ROS, lipid peroxide, glutathione, glutamate release into the extracellular space, and cystine uptake. As a result, inhibition of xCT by elastin was observed and also the release of glutamate and uptake of cystine were decreased. Furthermore, elastin treatment decreased intracellular glutathione while it increased intracellular Fe2+ , ROS, and lipid peroxides.

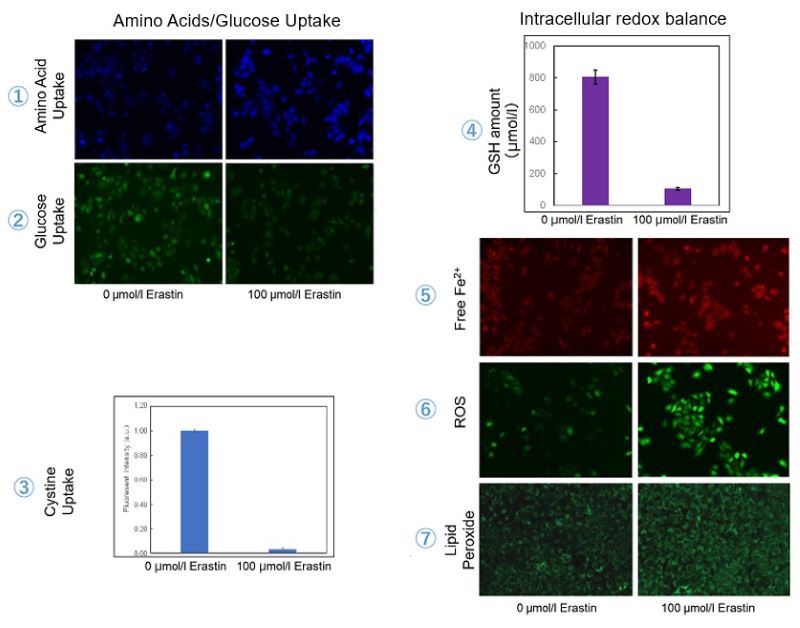
| 1 Amino Acid Uptake | : Amino Acid Uptake Assay Kit (Code: UP04) |
| 2 Glucose Uptake | : Glucose Uptake Assay Kit-Green (Code: UP02) |
| 3 Cystine Uptake | : Cystine Uptake Assay Kit (Code: UP05) |
| 4 Intracellular glutathione | : GSSG/GSH Quantification Kit (Code: G257) |
| 5 Intracellular labile Fe | : FerroOrange (Code: F374) |
| 6 Intracellular total ROS | : ROS Assay Kit -Highly Sensitive DCFH-DA- (Code: R252) |
| 7 Lipid Peroxides | : Liperfluo (Code: L248) |
Cell Line: A549
Incubation Conditions: 100 μmol/l Erastin/MEM, 37℃, 3h
Application and Reference Using This Kit
Literature used “GSSG/GSH Quantification Kit”
Diurnal Variation of cadmium-induced mortality in mice
N. Miura, Y. Yanagiba, K. Ohtani, M. Mita, M. Togawa, and T. Hasegawa, J. Toxicol. Sci., 2012, 37(1), 191
– Measuring object: Hepatic GSH
– Sample: Liver (Mice)
– Preparation of sample
1) Homogenize liver sample in 5% SSA for 30 seconds in ice-water bath.
2) Centrifuge the homogenates consisting of 100 mg liver in 1 ml (10%) at 8,000 x g for 10 min. at 4°C to remove proteins.
3) Assay with supernatants for GSH using GSSG/GSH Quantification Kit according to the manufacture’s instruction.
Effect of Oxidative Stress on Secretory Function in Salivary Gland Cells
K. Okabayashi, T. Narita, Y. Takahashi, H. Sugiya, (2012), Oxidative Stress – Environmental Induction and Dietary Antioxidants, Edited by Volodymyr I. Lushchak, ISBN 978-953-51-0553-4, Hard cover, 388 pages, Publisher: InTech
– Measuring object: GSH
– Sample: Parotid acinar cells
– Preparation of sample
1) Collect parotid acinar cells by centrifugation at 10,000 g for 15 s and immediately mixed with 160 ul of 10 mM HCl.
2) Freeze and thraw the mixture three times over, then mix with 40 ul of 5% SSA and then centrifuge at 8,000 g for 10 minutes.
3) Collect the supernatant and dilute twice.
Chloroplast DNA Replication Is Regulated by the Redox State Independently of Chloroplast Division in Chlamydomonas reinhardtii
Y. Kabeya, and S. Miyagishima, Plant Physiol., 2013, 161, 2102
– Measuring object: GSH and GSSG
– Sample: Chlamydomonas reinhardtii
– Preparation of sample
1) Collect Chlamydomonas reinhardtii cells by 1,000g for 5 min and wash with PBS.
2) Resuspended in 5% SSA solution and disrupted by sonication.
3) Collect the supernatants by 15,000g for 5 min.
4) Isolate chloroplst by 700g for 5 min and wash with 50 mM HEPES-KOH, pH 7.5, containing 300mM sorbitol.
5) Resuspend in 5% SSA solution and centrifuge at 15,000g for 5 min
Selected publications
ER Stress Cooperates with Hypernutrition toTrigger TNF-Depe. Cancer Cell. 2014;26:331-343.
Purpose: Determine indicator of ER Stress in Liver
Mechanisms of cadmium-inducedchronotoxicity in mice. The journal of toxicological. 2013;38:947-957.
Purpose: Determine the GSH/GSSG ratio in cadmium exposed Liver
Hepatitis C Virus Core Protein SuppressesMitophagy by Interacting with Parkin in the Context of MitochondrialDepolarization. The American Journal of Pathology. 2014;184:3026-3039.
Purpose: Measure mitochondrial oxidative status in liver
Possible involvement of glutathione balance disruption in dihydropyrazine-induced cytotoxicity on human hepatoma HepG2 cells.The journal of toxicological.2012;37:1065-1069.
Purpose: Investigate fluctuation of GSH/GSSG ratio in the cytotoxicity HepG2 Cells
Induction of heme oxygenase-1 contributes to survival of Mycobacterium abscessus in humanmacrophages-likeTHP-1cells.Redox Biology. 2015;4:328-339.
Purpose: Determine redox state in macrophages
Age-related changes in salivary biomarkers.Journal of Dental Sciences. 2014;9:85-90.
Purpose: Measure GSH/GSSG ratio in salivary
Protective effects of hydrogen sulfideanions against acetaminophen-induced hepatotoxicity in mice. The journal oftoxicological. 2015;40:837-841.
Purpose: Measure glutathione of Hepatocyte
References
1. N. Kubota, et al., A high-fat diet and multiple administration of carbon tetrachloride induces liver injury and pathological features associated with non-alcoholic steatohepatitis in mice. Clin Exp Pharmacol Physiol. 2013; 40(7): 422-30
2. T. Tomofuji, et al., Supplementation of broccoli or Bifidobacterium longum-fermented broccoli suppresses serum lipid peroxidation and osteoclast differentiation on alveolar bone surface in rats fed a high-cholesterol diet. Nutr Res. 2012; 32(4): 301-7
3. T. Miyayama., et al., Mitochondrial electron transport is inhibited by disappearance of metallothionein in human bronchial epithelial cells following exposure to silver nitrate. Toxicology. 2013; 8;305: 20-9.
4. H. Miwa, et al., Leukemia cells demonstrate a differen t metabolic perturbation provoked by 2-deoxyglucose. Oncol Rep. 2013; 29(5): 2053-7.
5. K. Unno, et al., Acute enhancement of non-rapid eye movement sleep in rats after drinking water contaminated with cadmium chloride. J Appl Toxicol. 2013 24.
6. Y. Ishihara, et al., Tributyltin induces oxidative stress and neuronal injury by inhibiting glutathione S-transferase in rat organotypic hippocampal slice cultures. Neurochem Int. 2012; 60(8): 782-90.
7. H. Miyamoto, et al., Thermophile-fermented compost as a possible scavenging feed additive to prevent peroxidation. J Biosci Bioeng. 2013; 116(2): 203-8.
8. E. Taniai, et al., Ochratoxin A induces karyomegaly and cell cycle aberrations in renal tubular cells without relation to induction of oxidative stress responses in rats. Toxicology Letters, October 2013.
9. G. Tian, et al., Ubiquinol-10 Supplementation Activates Mitochondria Functions to Decelerate Senescence in Senescence Accelerated Mice. Antioxidants & Redox Signaling, October 2013.
References
1) M. E. Anderson, "Determination of Glutathione and Glutathione Disulfide in Biological Samples", Methods in Enzymol., 1985, 113, 548.
2) M. A. Baker, G. J. Cerniglia and A. Zaman, "Microtiter Plate Assay for the Measurement of Glutathione and Glutathione Disulfide in Large Numbers of Biological Samples", Anal. Biochem., 1990, 190, 360.
3) C. Vandeputte, I. Guizon, I. Genestie-Denis, B. Vannier and G. Lorenzon, "A Micrototer Plate Assay for Total Glutathione and Glutathione Disulfide Contents in Cultured/isolated Cells: Performance Study of a New Miniaturized Protocol", Cell Biol. Toxicol., 1994, 10, 415.
4) S. A. McGrath-Morrow and J. Stahl, "Inhibition of Glutamine Synthetase in A549 Cells During Hyperoxia", Am. J. Respir. Cell Mol. Biol., 2002, 27, 99.
5) T. Sato, K. Seyama, Y. Sato, H. Mori, S. Souma, T. Akiyoshi, Y. Kodama, T. Mori, S. Goto, K. Takahashi, Y. Fukuchi, N. Maruyama and A. Ishigami, "Senescence Marker Protein-30 Protects Mice Lungs from Oxidative Stress, Aging, and Smoking", Am. J. Respir. Crit. Care Med., 2006, 174, 530.
6) M. L. Mulhern, C. J. Madson, A. Danford, K. Ikesugi, P. F. Kador and T. Shinohara, "The Unfolded Protein Response in Lens Epithelial Cells from Galactosemic Rat Lenses", Invest. Ophthalmol. Vis. Sci., 2006, 47(9), 3951.
7) N. Miura, Y. Yanagiba, K. Ohtani, M. Mita, and M. Togawa, "Diurnal Variation of Cadmium-induced Mortality in Mice", J. Toxicol. Sci., 2012, 37(1), 191.
8) K. Okabayashi, T. Narita, Y. Takahashi and H. Sugiya, "Effrct of Oxidative Stress on Secretory Function in Salivary Gland Cells", Oxidative Stress-Environmental Induction and Dietary Antioxidants, V. Lushchak, InTech, 2012, 189.
9) G. Tian, J. Sawashita, H. Kubo, S. Nishio, S. Hashimoto, N. Suzuki, H. Yoshimura, M. Tsuruoka, Y. Wang, Y. Liu, H. Luo, Z. Xu, M. Mori, M. Kitano, K. Hosoe, T. Takeda, S. Usami and K. Higuchi, "Ubiquinol-10 Supplementation Activates Mitochondria Functions to Decelerate Senescence in Senescence-Accelerated Mice", Antioxid. Redox Signal., 2014, 20(16), 2606.
10) H. Nakagawa, A. Umemura, K. Taniguchi, J. Font-Burgada, D. Dhar, H. Ogata, Z. Zhong, M. A. Valasek, E. Seki, J. Hidalgo, K. Koike, R. J. Kaufman and M. Karin, "ER Stress Cooperates with Hypernutrition to Trigger TNF-Dependent Spontaneous HCC Development", Cancer cell, 2014, 26(3), 331.
11) M. Sueyoshi, M. Fukunaga, M. Mei, A. Nakajima, G. Tanaka, T. Murase, Y. Narita, S. Hirata, and D. Kadowaki, "Effects of lactulose on renal function and gut microbiota in adenine-induced chronic kidney disease rats", Clinical and Experimental Nephrology., 2019,doi: 10.1007/s10157-019-01727-4.
12) S. Shiromizu, T. Yamauchi, N. Kusunose, N. Matsunaga, S. Koyanagi and S. Ohdo, Dosing Time-Dependent Changes in the Anti-tumor Effect of xCT Inhibitor Erastin in Human Breast Cancer Xenograft Mice', Biol. Pharm. Bull.., 2019, 42, (11), 1921-1925.
Q & A
-
Q
What kind of samples can be measured?
-
A
Tissue, plasma, red blood cells, and cell samples can be measured.
However, plasma samples are low in glutathione and may be difficult to measure depending on the sample.
-
Q
How many samples can I measure with this kit?
-
A
This kit is 200 tests (for 2 x 96-well plates).
When n=3, 18 samples can be assayed (when there is no coloration in the sample).
*If you do not separate samples (measure only total glutathione), 50 samples can be measured.
*If the sample is colored, a sample blank is required.
For details on how to measure a sample blank, please refer to the FAQ "How to measure a sample blank if the sample is colored".
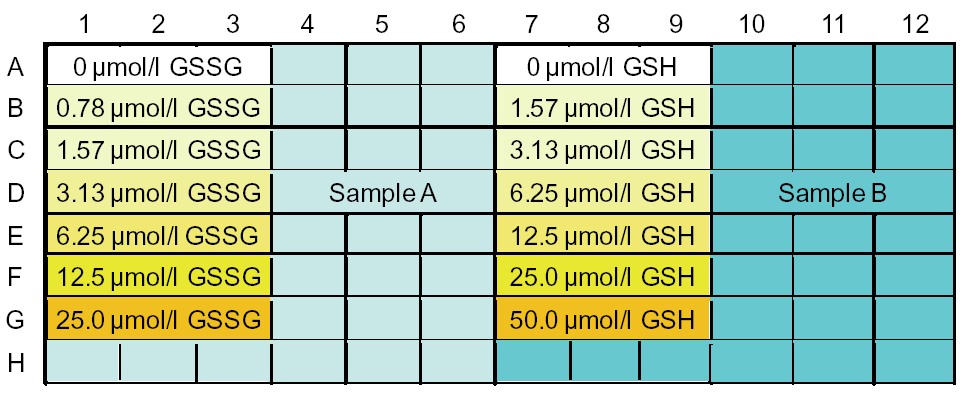
Sample layout example: Lanes 1-6 for GSSG concentration measurement and lanes 7-12 for total glutathione concentration measurement
-
Q
How to measure a sample blank if the sample is colored
-
A
If the sample has coloration, the sample blank can be subtracted by either of the following two methods.
(1) Kinetic method.
The sample blank is not affected because the calibration curve is created based on the slope of the calibration curve.
(2) When calculating glutathione concentration, instead of subtracting the O.D. blank, subtract the separately measured O.D. sample blank.
Glutathione (GSH, GSGG) = (O.D. sample - O.D. sample blank) / slope of the calibration curve
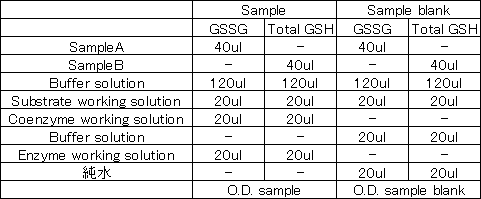
<How to measure O.D. sample blank>
Measure absorbance according to "4. Measurement" in the instruction manual.
In this case, do not add Coenzyme working solution or Enzyme working solution, Buffer solution and pure water are added.
-
Q
What does measuring the GSSG/GSH ratio reveal?
-
A
Glutathione normally exists in the reduced form (GSH) in the body but is converted to the oxidized form (GSSG) by stimuli such as oxidative stress. The ratio of GSH to GSSG is an indicator of oxidative stress.
-
Q
How do you calculate GSH levels?
-
A
From the total glutathione (GSH + GSSG) and GSSG concentrations obtained from the calibration curve, the GSH concentration is calculated using the following formula.
GSH Concentration = Total Glutathione Concentration - [GSSG Concentration] x 2
*One molecule of GSSG (oxidized glutathione) is equivalent to two molecules of GSH (reduced glutathione).
Therefore, the GSSG concentration is converted to GSH concentration by doubling the GSSG concentration.
-
Q
Is there a manufacturer or grade of 5-sulfosalicylic acid that you recommend?
-
A
There are no specific manufacturer recommendations. Please use reagent grade products.
-
Q
Does the masking reagent also mask GSH derived from GSSG?
-
A
After the addition of enzymes and coenzymes, GSH generated from GSSG reacts with DTNB before it reacts with the masking agent and returns to GSSG.
Therefore, even GSH generated from GSSG is not masked.
-
Q
Longer masking time (37°C, more than 1 hour) is a problem?
-
A
We have measured samples for up to 2 hours.
-
Q
What is the difference between this kit and the Total Glutathione Quantification Kit (Code: T419-10)?
-
A
The GSSG/GSH Quantification Kit (G257-10) can measure GSSG and GSH separately, which is not possible with the Total Glutathione Quantification Kit (T419-10).
-
Q
The amount of glutathione in my sample is high. Can I dilute the sample?
-
A
Dilute with 0.5% sulfosalicylic acid (SSA) solution.
The concentration of SSA in the sample should be 0.5 to 1% at the time of measurement.A high concentration of SSA can lower the pH during the reaction and affect the absorbance.
-
Q
Can the masking time (1 hour at 37°C) be omitted if only total glutathione is measured?
-
A
Optionally, you can omit it.
-
Q
What should I do if the coloration is weak?
-
A
The following three factors can be considered.
1. The measuring range of this kit is 0.5 μmol/L or higher. The amount of glutathione in the test sample may be lower.
Since samples outside the measuring range cannot be measured with this kit, please consider other methods such as the HPLC method.
If possible, reduce the dilution factor of the pretreatment or increase the sample volume to increase the concentration of the sample above the measuring range.
*Please note that the linearity of the calibration curve will not be obtained if the coloration time is prolonged to enhance the coloration.2. Substances that inhibit coloration may be included. For example, compounds that react with SH groups (maleimides) may affect the measurement.
Since these compounds cannot be removed by pretreatment, etc., measure the sample again without adding them to the sample.3. The pH during the reaction is low, which may cause chromogenic inhibition.
Check whether the SSA concentration is 1% or less at the time of measurement; if the SSA concentration is 1% or more, chromogenic inhibition will occur.
If other acids are used, neutralize to pH 7 with triethanolamine, etc., or dilute to an acid concentration of 1% or less before measuring.
-
Q
The measured value is negative. What could be the cause?
-
A
There are three possible causes
1. The amount of glutathione in the sample may be low.
Consider increasing the sample volume.
In addition, plasma may have a low glutathione content and may be difficult to measure.2. GSH may have decreased due to side reactions with amino groups during sample pretreatment.
It has been reported that GSH is reduced by the following side reactions under neutral conditions (Methods in Enzymology, 1985, 113, 548).
Therefore, sample pretreatment (5% SSA treatment) should be performed quickly.GSH + amino acid ⇔ γ-Glu-amino acid +CysH-Gly (amino group transfer)
GSH + H2O → Glu +CysH-Gly (hydrolysis)
GSH + GSH ⇔ γ-Glu-GSH +CysH-Gly (amino group transfer)3. GSH may have been oxidized to GSSG during sample preparation.
Perform sample pretreatment (5% SSA treatment) quickly as in 2.
Handling and storage condition
| 0-5°C | |
|
Danger / harmful symbol mark |
   
|
|---|---|