SOD Assay Kit - WST
SOD Activity Assay
- WST-1 based SOD inhibition assay
- Colorimetric microplate measurement
- Measures 100% inhibition by SOD
- pH-independent IC50 determination
- Low background noise measurement
-
Product codeS311 SOD Assay Kit - WST
| Unit size | Price | Item Code |
|---|---|---|
| 500 tests | Find your distributors | S311-10 |
| 500 tests | ・WST Solution ・Enzyme Solution ・Buffer Solution ・Dilution Buffer |
1 ml x 1 20 μl x 1 11 ml x 2 10 ml x 1 |
|---|
Description
Product Description
Superoxide dismutase (SOD), which catalyzes the dismutation of the superoxide anion (O2.–) into hydrogen peroxide and molecular oxygen, is one of the most important antioxidative enzymes. In mammals, cytosolic SOD has a greenish color and consists of two subunits, one containing copper and the other zinc (Cu/Zn-SOD). Mitochondrial and bacterial SOD has a reddish-purple color and contains manganese (Mn-SOD). E. coli has Mn-SOD and SOD containing iron (Fe-SOD). Several direct and indirect methods have been developed to determine SOD activity. An indirect method using nitrotetrazolium blue is often used because of its convenience. However, there are several disadvantages to this method, such as poor water solubility of the formazan dye and its reaction with the reduced form of xanthine oxidase. Although cytochrome C is also commonly used for SOD activity detection, its reactivity with superoxide is too high to determine low levels of SOD activity.
SOD Assay Kit-WST allows a very convenient and highly sensitive SOD assay by utilizing Dojindo’s highly water-soluble tetrazolium salt, WST-1 (2-(4-iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfo-phenyl)-2H-tetrazolium, monosodium salt), which produces a water-soluble formazan dye upon reduction with a superoxide anion (Fig. 1). The absorption spectrum is shown in Fig. 2. WST-1 is 70 times less reactive with superoxide anion than cytochrome C; therefore, highly sensitive SOD detection is possible and samples can be diluted with buffer to minimize background problems. WST-1 does not react with the reduced form of xanthine oxidase; therefore, even 100% inhibition with SOD is detectable. The rate of WST-1 reduction by superoxide anion is linearly related to the xanthine oxidase activity and is inhibited by SOD (see figure below). Therefore, the IC50 (50% inhibition concentration) of SOD or SOD-like materials can be determined using colorimetric methods.
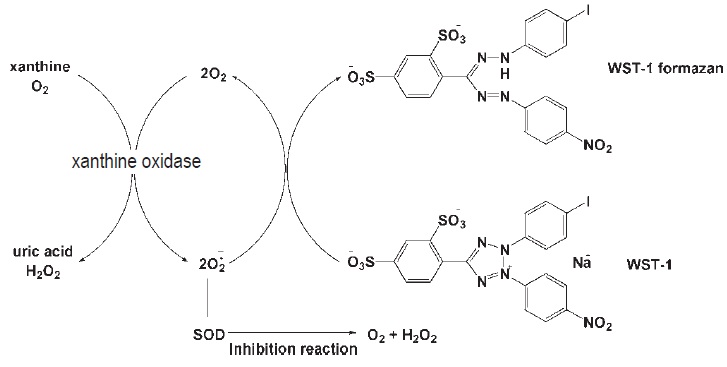
Fig. 1 SOD Inhibition assay mechanism
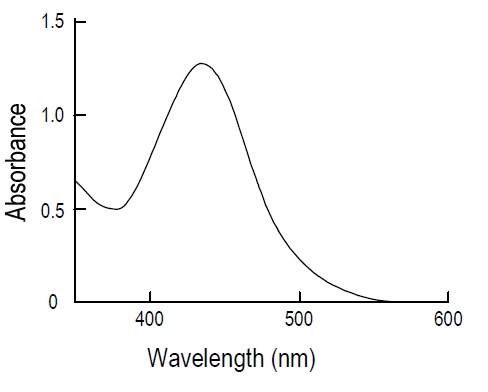
Fig. 2 Absorption spectrum of WST-1 formazan
| Developer | Dojindo Molecular Technologies, Inc. |
|---|
Manual
Technical info

 a)After the addition of enzyme working solution, the mixed solution generates superoxide. Use a multi-channel pipette to add the enzyme working solution to minimize the reaction time lag.
a)After the addition of enzyme working solution, the mixed solution generates superoxide. Use a multi-channel pipette to add the enzyme working solution to minimize the reaction time lag.
b)If the microplate reader has a temperature control function, incubate the plate on the microplate holder at 37°C.
Recent Publications
| Samples from | Treatments | References |
|---|---|---|
| hESCs | SOD-1 overexpressing | Amyotrophic Lateral Sclerosis Model Derived from Human Embryonic Stem Cells Overexpressing Mutant Superoxide Dismutase 1 T. Wada, et al., Stem Cells Trans Med, 1, 396(2012) |
| mouse heart, liver | tetrathiomolybdate | Copper chelation by tetrathiomolybdate inhibits lipopolysaccharide-induced inflammatory responses in vivo H. Wei, et al., Am J Physiol Heart Circ Physiol, 301, H712(2011) |
| MEF cells | presenilin knock-out | Presenilins Promote the Cellular Uptake of Copper and Zinc and Maintain Copper Chaperone of SOD1-dependent Copper/Zinc Superoxide Dismutase Activity M. A. Greenough, et al., J Biol Chem, 286, 9776(2011) |
| mouse lung | SOD3 knockout, overexpressing | Extracellular superoxide dismutase protects against pulmonary emphysema by attenuating oxidative fragmentation of ECM H. Yao, et al., PNAS, 107, 15571(2010) |
| mouse liver Nrf2 |
nuclear factor-E2-related factor-2 | Deletion of nuclear factor-E2-related factor-2 leads to rapid onset and progression of nutritional steatohepatitis in mice H. Sugimoto, et al., Am J Physiol Gastrointest Liver Physiol, 298, G283(2010) |
| bacteria (Francisella strains) |
gallium-transferrin | Gallium Disrupts Iron Uptake by Intracellular and Extracellular Francisella Strains and Exhibits Therapeutic Efficacy in a Murine Pulmonary Infection Model O. Olakanmi, et al., Antimicrob Agents Chemother, 54, 244(2010) |
Preparation of Various Sample Solution
Cells (Adherent cells: 9×106 cells, Leukocytes: 1.2 x107 cells)
1. Harvest cells with a scraper, centrifuge at 2,000 g for 10 min at 4ºC, and discard the supernatant.
2. Wash the cells with 1 ml PBS and centrifuge at 2,000 g for 10 min at 4ºC. Discard the supernatant. Repeat this step.
3. Break cells using the freeze-thaw method (-20ºC for 20 min, then 37ºC bath 10 min, repeat twice).
4. Add 1 ml PBS. If necessary, sonicate the cell lysate on an ice bath (60 W with 0.5 sec interval for 15 min).
5. Centrifuge at 10,000 g for 15 min at 4ºC.
Plant or Vegetable (200 mg)
1. Add 1 ml distilled water, and homogenize the sample using a homogenizer with beads.
2. Filter the homogenate with paper filter, and lyophilize the filtrate.
3. Measure the weight of the lyophilized sample, and dissolve with 0.1 M phosphate buffer (pH 7.4) to prepare sample solution.
Tissue (100 mg)
1. Wash the tissue with saline to remove as much blood as possible. Blot the tissue with paper towels and then measure its weight.
2. Add 400-900 μl sucrose buffer (0.25 M sucrose, 10 mM Tris, 1 mM EDTA, pH 7.4) and homogenize the sample using Teflon homogenizer. If necessary, sonicate the homogenized sample on an ice bath (60W with 0.5 second intervals for 15 min).
3. Centrifuge the homogenized sample at 10,000 g for 60 min at 4ºC, and transfer the supernatant to a new tube.
Tea (antioxidant activity detection)
1. Add 60 ml boiled water to 10 g of tea, and leave it for 2.5 min.
2. Filter the extract with paper filter and then filter again with a 0.45 μm membrane filter.
3. Dilute the filtrate with distilled water to prepare sample solution.
Erythrocytes or Plasma
1. Centrifuge 2-3 ml of anticoagulant-treated blood (such as heparin 10 U/ml final concentration) at 600 g for 10 min at 4°C.
2. Collect the supernatant and dilute it with saline to use as a plasma sample.
3. Add the same volume of saline as the original sample (in step 1) to the pellet and suspend.
Transfer the 0.4 ml of the suspension to the 10 ml glass centrifuge tube and add 9.6 ml of saline.
4. Centrifuge the suspension at 600 g for 10 min at 4ºC.
5. Discard the supernatant, then add the 10 ml of saline to the pellet and suspend. Centrifuge the suspension at 600 g for 10 min at 4ºC.
6. Repeat step 5 twice.
7. Discard the supernatant, then add 4 ml of distilled water to the pellet and suspend(Erthrocytes are hemolyzed and SOD are released.).
Add 1 ml of ethanol and 0.6 ml of chloroform to the hemolysate.
8. Shake vigorously the stopped test tube with a shaker for 15 min at 4°C.
9. Centrifuge 600 g for 10 min at 4ºC, then transfer the upper water-ethanol phase to a new tube.
10. Take 0.1 ml of water-ethanol phase and mix with 0.7 ml of distilled water to prepare the erythrocytes sample.
*If the erythrocytes sample needs to be diluted, use 0.25% ethanol to dilute.
Extracellular SOD (EC-SOD)
1. Prepare a 0.5 ml volume of Con A-sepharose column equilibrated with PBS.
2. Apply supernatant of a tissue homogenate on the column, and leave the column for 5 min at room temperature.
3. Add total 10 ml PBS to wash the column.
4. Add 1 ml of 0.5 M α-methylmannoside/PBS, and collect the eluate. Repeat 5 times.
5. Use the eluate for the SOD assay without dilution. If the SOD activity is high enough, dilute the eluate with PBS.
Wine (antioxidant activity detection)
1. Filter wine with a 0.45 μm membrane filter.
2. Dilute the filtrate with distilled water to prepare sample solution.
Data
Inhibition curve by WST Method
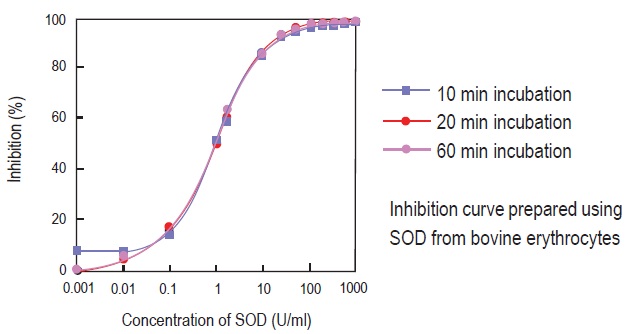
Fig. 5 Inhibition curve prepared by different data acquisition times
References
1) H. Ukeda, A. K. Sarker, D. Kawana and M. Sawamura, "Flow-Injection Assay of Superoxide Dismutase Based on the Reduction of Highly Water-Soluble Tetrazolium", Anal. Sci., 1999, 15, 353.
2) H. Ukeda, D. Kawana, S. Maeda and M. Sawamura, "Spectrophotometric Assay for Superoxide Dismutase Based on the Reduction of Highly Water-soluble Tetrazolium Salts by Xanthine-Xanthine Oxidase", Biosci. Biotechnol. Biochem., 1999, 63, 485.
3) H. Ukeda, T. Shimamura, M. Tsubouchi,Y. Harada, Y. Nakai and M. Sawamura, "Spectrophotometric Assay of Superoxide Anion Formed in Maillard Reaction Based on Highly Water-soluble Tetrazolium Salt", Anal. Sci., 2002, 18, 1151.
4) N. Tsuji, N. Hirayanagi, M. Okada, H. Miyasaka, K. Hirata, M. H. Zenk and K. Miyamoto, "Enhancement of Tolerance to Heavy Metals and Oxidative Stress in Dunaliella Tertiolecta by Zn-induced Phytochelatin Synthesis", Biochem. Biophys. Res. Commun., 2002, 293, 653.
5) A. Sakudo, D. C. Lee, K. Saeki, Y. Nakamura, K. Inoue, Y. Matsumoto, S. Itohara and T. Onodera, "Impairment of Superoxide Dismutase Activation by N-Terminally Truncated Prion Protein (PrP) in PrP-deficient Neuronal Cell Line", Biochem. Biophys. Res. Commun., 2003, 308, 660.
6) J.-H. Zhu, X. Zhang, J. P. Mcclung and X. G. Lei, "Impact of Cu,Zn-Superoxide Dismutase and Se-Dependent Glutathione Peroxidase-1 Knockouts on Acetaminophen-Induced Cell Death and Related Signaling in Murine Liver", Exp. Biol. Med., 2006, 231, 1726.
7) H. R. Rezvani, S. Dedieu, S. North, F. Belloc, R. Rossignol, T. Letellier, H. de Verneuil1, A. Taieb1, F.Mazurier, "HIF-1α: a Key Ffactor in the Keratinocyte Response to UVB Exposure", J. Biol. Chem., 2007, 10, 1074.
8) S. Goldstein and G. Czapski, "Comparison Between Different Assays for Superoxide Dismutase-like Activity", Free Rad. Res. Comms., 1991, 12, 5.
9) R. H. Burdon, V. Gill and C. Rice-Evans, "Reduction of a Tetrazolium Salt and Superoxide Generation in Human Tumor Cells (HeLa)", Free Rad. Res. Commun., 1993,18, 369.
10) Y. Sakurai, I. Anzai and Y. Furukawa, "A Primary Role for Disulfide Formation in the Productive Folding of Prokaryotic Cu,Zn-superoxide Dismutase", J. Biol. Chem., 2014, 289(29), 20139.
11) A. Weichert, A. S. Besemer, M. Liebl, N. Hellmann, I. Koziollek-Drechsler, P. Ip, H. Decker, J. Robertson, A. Chakrabartty, C. Behl and A. M. Clement, "Wild-type Cu/Zn superoxide dismutase stabilizes mutant variants by heterodimerization", Neurobiol. Dis., 2014, 62, 479.
12) J. M. Hartney, T. Stidham, D. A. Goldstrohm, R. E. Oberley-Deegan, M. R. Weaver, Z. Valnickova-Hansen, C. Scavenius, R. K.P. Benninger, K. F. Leahy, R. Johnson, F. Gally, B. Kosmider, A. K. Zimmermann, J. J. Enghild, E. Nozik-Grayck and R. P. Bowler, "A common polymorphism in extracellular superoxide dismutase affects cardiopulmonary disease risk by altering protein distribution", Circ Cardiovasc Genet, 2014, 7(5), 659.
13) J. Wu, Y. Sun, Y. Zhao, J. Zhang, L. Luo, M. Li, J. Wang, H. Yu, G. Liu, L. Yang, G. Xiong, J. Zhou, J. Zuo, Y. Wang and J. Li, "Deficient plastidic fatty acid synthesis triggers cell death by modulating mitochondrial reactive oxygen species", Cell Res., 2015, 25(5), 621.
14) E. Harunari, C. Imada, Y. Igarashi, "Konamycins A and B and Rubromycins CA1 and CA2, Aromatic Polyketides from the Tunicate-Derived Streptomyces hyaluromycini MB-PO13T'", J. Nat. Prod., 2019, 82, (6), 1609-1615.
15) N. Kajihara, D. Kukidome, K. Sada, H. Motoshima, N. Furukawa, T. Matsumura, T. Nishikawa and E. Araki, "Low glucose induces mitochondrial reactive oxygen species via fatty acid oxidation in bovine aortic endothelial cells", J Diabetes Investig, 2017, 8, (6), 750.
16) H- J. Kim, H- J. Kim, J. Jeon, K- C. Nam, K- S. Shim, J- H. Jung, K. S. Kim, Y. Choi, S- H. Kim, A. Jang, Comparison of the quality characteristics of chicken breast meat from conventional and animal welfare farms under refrigerated storage', Poultry Science., 2020, 99, 1788-1796.
17) T. Shoji, S. Masumoto, N. Moriichi, Y. Ohtake, T. Kanda, Administration of Apple Polyphenol Supplements for Skin Conditions in Healthy Women: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial', Nutrients., 2020, 12, (1071), .
18) Y. Hirata, Y. Ito, M. Takashima, K Yagy, K. Oh-Hashi, H. Suzuki, K. Ono, K. Furuta and M. Sawada, Novel Oxindole-Curcumin Hybrid Compound for Antioxidative Stress and Neuroprotection.', ACS Chem. Neurosci.., 2020, 11, (1), 76-85.
Q & A
-
Q
What is the definition of a Unit?
-
A
One unit is defined as a point where a sample gives 50% inhibition of a colorimetric reaction between reactive dye (such as cytochrome C, WST-1, nitro-tetrazolium blue or XTT) and superoxide anion. For example, if the O.D. of a sample that does not contain any SOD is 1.0, another sample that gives 0.5 O.D. is defined as having 1 unit of SOD activity. You can use this unit to determine the SOD activity of your sample. Therefore, SOD activities determined using different dyes or methods are not comparable with each other.
-
Q
Can I use standard SOD to determine SOD activity in sample solutions?
-
A
Yes, you can. Prepare a inhibition curve (typical inhibition curve, and determine SOD activity in the sample solution. SOD bovine erythrocytes (CAS# 9054-89-1, EC 1.15.1.1) can be purchased from Sigma (catalog# S7571).
-
Q
Can I use a kinetic method to determine SOD activity?
-
A
Yes, you can use a kinetic method for SOD assay. Since the rate of the color development remains the same for up to 20 minutes, measure the slope for 5 minutes during this linear phase.
-
Q
The sample has color. Can I still use this sample?
-
A
Yes, you can still use the sample. Diluting the sample will minimize the interference. Subtract the O.D. of blank 2 from the O.D. of the sample to cancel out the background color. However, if the SOD activity in the sample is low, it may not be measurable.
-
Q
How do I prepare more Dilution buffer?
-
A
Dilution buffer is PBS. Please prepare the Dilution buffer with following concentrations; 137 mM NaCl, 2.7 mM KCl, 1.47 mM KH2PO4, 8.1 mM Na2HPO4, pH 7.4.
-
Q
Can I determine Mn-SOD and Cu/Zn-SOD independently using this kit?
-
A
Yes. In order to measure Mn-SOD activity, it is necessary to block the Cu/Zn-SOD activity using potassium cyanide (KCN). Adding 1 mM KCN to samples can block Cu/Zn-SOD activity completely. To measure Cu/Zn-SOD activity, measure the total SOD activity with and without KCN, and then subtract the Mn- SOD activity from total SOD activity.
-
Q
Can Mn-SOD, Cu, Zn-SOD, and EC (extracellular)-SOD measurement be differentiated?
-
A
Mn-SOD can be measured by blocking the Cu/Zn-SOD and EC-SOD activity using potassium cyanide (KCN) or Diethyldithiocarbamate (DDC).
[Measurement Example] ・Add 1-10 mmol/l KCN or DDC to the sample solution and incubate at room temperature for 5 minutes.
・Measure SOD-activity and follow the SOD Assay Kit-WST Technical Manual.
*Caution*
・Optimization is required for the concentration of KCN or DDC and the incubation time by sample. Please refer to the references below.
・When calculating the unit (U), please consider the dilution ration of the sample prepared by adding KCN or DDC.<Reference using KCN (potassium cyanide)>
1) Greenough MA, Volitakis I, Li QX, Laughton K, Evin G, Ho M, Dalziel AH, Camakaris J, Bush AI, “Presenilins promote the cellular uptake of copper and zinc and maintain copper chaperone of SOD1-dependent copper/zinc superoxide dismutase activity”, J Biol Chem., 2011, 286, 9776.2) Hajime Fujimoto, Jun-ichi Taguchi, “Manganese superoxide dismutase polymorphism affects the oxidized low-density lipoproteininduced apoptosis of macrophages and coronary artery disease.”, European Heart Journal. 2008. 29, 1267.
3) A. Dacanay, S. C. Johnson, R. Bjornsdottir, R. O. Ebanks, N. W. Ross, M. Reith, R. K. Singh, J. Hiu, and L. L. Brown, “Molecular Characterization and Quantitative Analysis of Superoxide Dismutases in Virulent and Avirulent Strains of Aeromonas salmonicida subsp. salmonicida” Journal of Bacteriology, 2003, 185 (15), 4336.
<Reference using DDC (Diethyldithiocarbamate)>
1) Heikkila RE, Cabbat FS, Cohen G. “In vivo inhibition of superoxide dismutase in mice by diethyldithiocarbamate”, J Biol Chem., 1976, 251, 2182.
-
Q
How long can I store the sample?
-
A
A sample stored in a freezer at -80ºC is stable for 1 month.
-
Q
Can I measure the levels of superoxide anion using this kit?
-
A
No. However, you could simply use WST-1, instead of this kit, to measure superoxide. You would need a standard to determine the amount of superoxide in sample solution. Since superoxide is not stable and reacts with various materials, it might be difficult to determine the total amount of superoxide generated in the system. The xanthine-xanthine oxidase system in this kit can be used as a standard for measuring the relative amount of superoxide production in each sample.
Handling and storage condition
| 0-5°C |