General Information
Glutathione (γ-L-glutamyl-L-cysteinylglycine) is a tripeptide compound which exists in a body, and it is involved in antioxidation, drug metabolism, and others as an enzyme substrate of glutathione peroxidase, glutathione S-transferase, thiol transferase, and so on. Glutathione is usually presented as a reduced form (GSH), but GSH is converted into an oxidized form (GSSG) by stimulation of oxidative stress. Therefore, the ratio of GSH and GSSG has been focused as an index of oxidative stress.
The GSSG/GSH Quantification Kit contains the reagent for GSH masking. GSH in a sample solution can be removed by adding the Masking reagent. Therefore, GSSG in the sample solution can be selectively determined by measuring the absorption (λmax = 412 nm) derived from a colorimetric reaction of DTNB (5,5’-dithiobis (2-nitrobenzoic acid)) coupled with the enzymatic recycling system. The quantity of GSH can also be determined by subtracting the amount of GSSG from the total amount of glutathione.
The detection ranges of total glutathione and GSSG using in this kit are from 0.5 μmol/l to 50 μmol/l and from 0.5 μmol/l to 25 μmol/l, respectively.
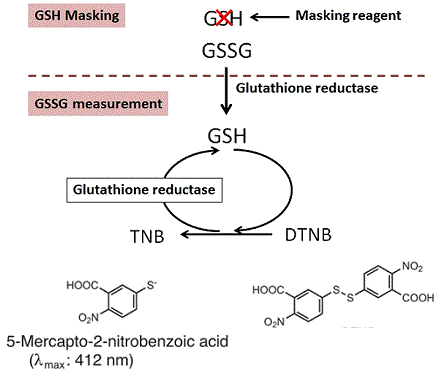
Fig.1 Principle of GSSG/GSH detection
Kit Contents
| Enzyme solution | 50 μl ×1 |
| Buffer solution | 60 ml ×1 |
| Standard GSH | ×1 |
| Masking reagent | 20 μl ×1 |
| Coenzyme | ×2 |
| Substrate (DTNB) | ×4 |
| Standard GSSG | ×1 |
Storage Condition
Store at 0-5oC. Use the kit after returning to room temperature.
Required Equipment and Materials
Equipment
-
- Plate reader (405 or 415 nm filter)
- 20 μl and 200 μl pipettes, a multi channel pipette
- 15 ml conical tube
-
- 96-well microplate
- Incubator
- Disposable syringes
Materials
-
- 5-Sulfosalicylic acid (SSA) solution
-
- Ethanol
- 5-Sulfosalicylic acid is NOT included in this kit.
Precaution
- Use the kit after returning to room temperature.
- Masking reagent is lachrymatory and irritating. Handle the reagent in a chemical hood.
- Triplicate measurement per sample is recommended to obtain accurate data.
- Since the reaction starts immediately after the addition of Enzyme/coenzyme working solution to the well, use a multichannel pipette to avoid the reaction time lag of each well.
- If a concentration range of total glutathione of a sample solution is not known, prepare multiply diluted sample solutions.
- This kit contains glass bottles with an aluminum cap. Please handle carefully.
- Prepared working solution can not store.
Example for Sample Preparation
Tissue (100 mg)
- Homogenize the tissue in 0.5-1.0 ml of 5% SSA.
- Centrifuge the homogenized tissue sample at 8,000 xg for 10 minutes.
- Transfer the supernatant to a new tube and add double-deionized H2O (ddH2O) to reduce the SSA concentration to 0.5% for the assay.
Plasma
- Centrifuge an anticoagulant-treated blood at 1,000 x g for 10 minutes at 4°C.
- Transfer the top layer of plasma to a new tube. Add 5% SSA (half volume of plasma) to the tube. (ex. 100 µl of 5% SSA for 200 µl of the plasma)
- Centrifuge at 8,000 x g for 10 minutes at 4°C.
- Transfer the supernatant to a new tube and add ddH2O to reduce the SSA concentration to 0.5 % for the assay.
Erythrocytes
- Centrifuge an anticoagulant-treated blood at 1,000 xg for 10 minutes at 4°C.
- Discard the supernatant and the white buffy coat.
- Add 4 times volume of 5% SSA to the erythrocytes to lysis.
- Centrifuge at 8,000 x g for 10 minutes at 4°C.
- Transfer the supernatant to a new tube, and add ddH2O to reduce the SSA concentration to 0.5% for the assay.
Cells (1x107 cells)
- Collect cells by centrifugation at 200 x g for 10 minutes at 4oC. Discard the supernatant.
- Wash the cells with 300 μl of PBS and centrifuge at 200 x g for 10 minutes at 4°C. Discard the supernatant.
- Add 80 μl of 10 mmol/l HCl, and lyse cells by freezing and thawing twice.
- Add 20 μl of 5% SSA and centrifuge at 8,000 x g for 10 minutes.
- Transfer the supernatant to a new tube, and add ddH2O to reduce the SSA concentration to 0.5% for the assay.
Preparation of Solution
200 μmol/l GSH standard solution
Add 2.0 ml of 0.5 % SSA solution to a Standard GSH vial and dissolve.
- Since the Standard GSH is packed under reduced pressure, open after adding 0.5 % SSA solution with a syringe through a rubber septum.
- Store the GSH standard solution at -20°C, the solution is stable for 2 months.
100 μmol/l GSSG standard solution
Add 2.0 ml of 0.5 % SSA solution to the Standard GSSG vial and dissolve.
- Since the Standard GSSG is packed under reduced pressure, open after adding 0.5 % SSA solution with a syringe through a rubber septum.
- Store the GSSG standard solution at -20°C, the solution is stable for 2 months.
Masking solution
Add 180 μl of ethanol to a Masking reagent vial and mix by pipetting.
- Masking reagent is lachrymatory and irritating. Handle the reagent in a chemical hood.
- Masking reagent may be yellow to reddish brown colored.
- Shake off the tube briefly before opening to remove all contents from the tube wall and inside the cap.
- Store the Masking solution at 0-5°C, the solution is stable for 2 months.
- When storing Masking solution at 0-5°C, tightly seal the container with Parafilm to prevent ethanol from volatilizing.
Substrate working solution
- Add 1.2 ml of Buffer solution to a Substrate (DTNB) vial and dissolve.
- Please make sure that all of the Substrate is dissolved completely. We recomend using a vortex mixer or an ultra-sonic bath.
- The solution prepared in step 1. is stable for 2 months at -20 °C.
- Transfer all of the solution prepared in step 1) to a 15 ml conical tube and dilute it with 2.4 ml of Buffer solution
(total volume: 3.6 ml).- Use 2 vials of Substrate for a 96-well microplate (total volume: 7.2 ml).
- The solution prepared in step 2. can’t be stored.
Enzyme/coenzyme working solution
- Pipette to mix Enzyme solution, and take 20 μl to a 15 ml conical tube to dilute with 4 ml of Buffer solution.
- Enzyme solution may be on the wall or inside of the tube cap. Please shake down before opening. Do not use a vortex mixer to prepare the working solution.
- The solution prepaed in setp 1. is stable for 2 months at 0-5 °C.
- Transfer 2.4 ml of the solution prepared in step 1. to a new 15 ml conical tube.
- Add 2.4 ml of ddH2O to a Coenzyme vial and dissolve.
- Since the Coenzyme is packed under reduced pressure, open after adding ddH2O with a syringe through a rubber septum.
- The solution prepared in step 3. is stable for 2 months at -20 °C.
- Transfer all of the solution prepared in step 3) to a 15 ml conical tube in step 2. and dilute it with 2.4 ml of Buffer solution (total volume: 7.2 ml).
- The solution prepared in step 4. can’t be stored.
General Protocol
1. Sample preparation
- Prepare two sets of sample solutions (200 μl each x 2) if both GSH and GSSG have to be determined.
- For GSSG measurement:
Add 200 μl of the sample to microtube, 4 μl of Masking solution, and mix with a vortex mixer. [Sample (GSSG)] - For total glutathione measurement: Prepare 200 μl sample. [Sample (GSH)]
- If a concentration range of total glutathione in a sample solution is not known, prepare multiply diluted sample solutions.
2. Preparation of GSSG standard solution
- Mix 100 μl of 100 μmol/l GSSG standard solution and 300 μl of 0.5% SSA solution in a microtube to prepare a 25 μmol/l GSSG standard solutions. Prepare the following GSSG standard solutions by serial dilution using 0.5% SSA solution: 25.0, 12.5, 6.25, 3.13, 1.57, 0.78 and 0 μmol/l.
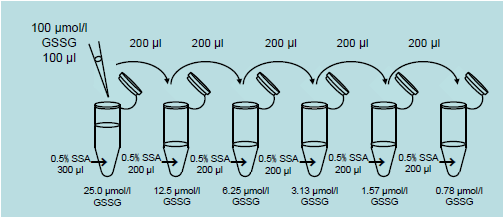
- Add 4 μl of Masking solution to all prepared GSSG standard solutions, and mix with a vortex mixer.
3. Preparation of GSH standard solution
Mix 100 μl of 200 μmol/l GSH standard solution and 300 μl of 0.5% SSA solution in a microtube to prepare a 50 μmol/GSH standard solution. Prepare the following GSH standard solutions by serial dilution using 0.5% SSA solution: 50.0, 25.0, 12.5, 6.25, 3.13, 1.57 and 0 μmol/l.
4. Measurement
- Add 40 μl of GSSG standard solution, GSH standard solution, Sample (GSSG), or Sample (GSH) to each well.
- Triplicate measurement per sample is recommended to obtain a ccurate data.
- Add 60 μl of Buffer solution to each well.
- Incubate the plate at 37 oC for 1 hour.
- Use a well cap for the microplate to prevent evaporation of the solution during the incubation.
- Add 60 μl of Substrate working solution to each well.
- Add 60 μl of Enzyme/coenzyme working solution to each well.
- Since the reaction starts immediately after the addition of Enzyme/coenzyme working solution, use a multichannel pipette to
avoid the reaction time lag of each well.
1 2 3 4 5 6 7 8 9 10 11 12 A 0 μmol/l GSSG Sample 2 (GSSG) 0 μmol/l GSH Sample 2 (GSH) B 0.78 μmol/l GSSG Sample 3 (GSSG) 1.57 μmol/l GSH Sample 3 (GSH) C 1.57 μmol/l GSSG Sample 4 (GSSG) 3.13 μmol/l GSH Sample 4 (GSH) D 3.13 μmol/l GSSG Sample 5 (GSSG) 6.25 μmol/l GSH Sample 5 (GSH) E 6.25 μmol/l GSSG Sample 6 (GSSG) 12.5 μmol/l GSH Sample 6 (GSH) F 12.5 μmol/l GSSG Sample 7 (GSSG) 25.0 μmol/l GSH Sample 7 (GSH) G 25.0 μmol/l GSSG Sample 8 (GSSG) 50.0 μmol/l GSH Sample 8 (GSH) H Sample 1 (GSSG) Sample 9 (GSSG) Sample 1 (GSH) Sample 9 (GSH) Fig. 3 An example of plate arrangement (n=3) - Since the reaction starts immediately after the addition of Enzyme/coenzyme working solution, use a multichannel pipette to
- Incubate at 37°C for 10 minutes.
- If you choose a kinetics method, select “Kinetic” mode.
- Since the O.D. increases linearly over 10 minutes after the start of reaction, glutathione concentration can be determined by using kinetic method or pseudo-end point method. (measurement of the O.D. at certain time points between 5 and 10 minutes without a stopping reaction)
- Read the absorbance at 405 or 415 nm using a microplate reader.
- Determine the concentration of GSSG in the sample [Sample (GSSG)] using the GSSG calibration curve. [GSSG concentration]
- Determine the concentration of total glutathione (GSH + GSSG) in the sample [Sample (GSH)] using the GSH calibration curve. [total glutathione concentration]
-

1) Add 40 μl of GSSG standard solution, GSH standard solution,Sample (GSSG), or Sample (GSH) to each well.
-

2) Add 60 μl of Buffffer solution to each well.
-

3) Incubate the plate at 37ºC for 1 hour.
-

4)-5) Add 60 μl of Substrate working solution, 60 μl of Enzyme/coenzyme working solution to each well.
-
7) Read the absorbance at 405 or 415 nm using a microplate reader.
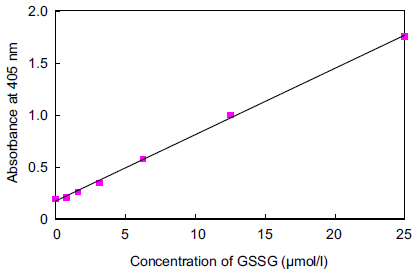
Fig.4 Typical calibration curve of GSSG
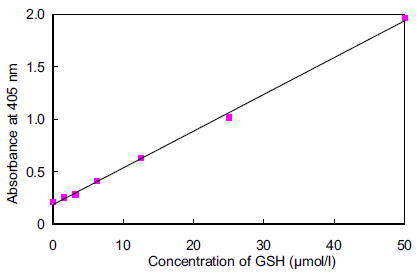
Fig.5 Typical calibration curve of GSH
The concentration of glutathione can be determined by either Kinetic method or Pseudo-end point method.
Pseudo-end point method: Glutathione (GSH, GSSG) = (O.D.sample - O.D.blank a)) / slope b) Kinetic method: Glutathione (GSH, GSSG) = ( slopesamplec) - slopeblank a), c)) / slope b)
- a) Apply the O.D. values from A1-A3 wells or A7-A9 wells in Fig. 3.
- b) The slope value is determined from the calibration curve of the pseudo-end point or the kinetic method.
- c) The slope values for sample and blank are determined from kinetic reactions of each sample and blank.
- In case the original samples are diluted for this assay, multiply the determined value from above equation by the dilution ratio to calcurate the glutathione concentration of original sample.
-
- GSH concentration is calculated from the [GSSG concentration] obtained in step 8. and the [total glutathione concentration] obtained in step 9., using the following equation.
GSH concentration = [total glutathione concentration] – [GSSG concentration] x 2
Interference
Reducing agents such as ascorbic acid, β-mercaptoethanol, dithiothreitol (DTT) and cysteine, or thiol reactive compounds such as maleimide compounds, interfere with the glutathione assay. Therefore, these materials should be avoided during the sample preparation.
Frequently Asked Questions / Reference
G257: GSSG/GSH Quantification Kit
Revised Jul., 29, 2024


 Hidden sections will not be printed.
Hidden sections will not be printed.

