|
In neurodegeneration, dysfunctional mitochondria contribute to oxidative stress and energy deficits in neurons. Lysosomes, responsible for the disposal of cellular waste, may be impaired, leading to the accumulation of damaged organelles, including mitochondria. At the same time, abnormal lipid metabolism and accumulation of lipid droplets have been implicated in neurodegenerative diseases, potentially exacerbating neuronal dysfunction and contributing to disease progression. The intricate relationships between dysfunctional mitochondria, impaired lysosomal function and abnormal lipid metabolism underscore the complex pathophysiology of neurodegeneration.
|
-
Messenger RNA transport on lysosomal vesicles maintains axonal mitochondrial homeostasis and prevents axonal degeneration
Click here for the original article: Raffaella De Pace, et. al., Nature Neuroscience, 2024.
Point of Interest
- Lysosome–kinesin adaptor related complex (BORC) KO depletes axonal mRNAs mainly encoding ribosomal and mitochondrial/oxidative phosphorylation proteins.
- This depletion leads to mitochondrial defects and ultimately to axonal degeneration in neurons.
- A mechanistic connection of BORC deficiency may accelerate common neurodegenerative disorders.
-
APOE4/4 is linked to damaging lipid droplets in Alzheimer’s disease microglia
Click here for the original article: Michael S. Haney,et. al., Nature, 2024.
Point of Interest
- Lipid droplet-associated enzyme ACSL1-positive microglia was most abundant in patients with AD having the APOE4/4 genotype.
- In microglia, fibrillar Aβ induces ACSL1 expression, triglyceride synthesis and lipid droplet accumulation depending on APOE.
- Conditioned media from lipid droplet-containing microglia lead to Tau phosphorylation and neurotoxicity depending on APOE in neurons.
-
Loss of WIPI4 in neurodegeneration causes autophagy-independent ferroptosis
Click here for the original article: Ye Zhu, et. al., Nature Cell Biology, 2024.
Point of Interest
- WIPI4 deficiency causes β-Propeller protein-associated neurodegeneration, which induces ferroptosis via an autophagy-independent mechanism in cell culture and in zebrafish.
- WIPI4 depletion increases the localization of ATG2A at ER-mitochondrial contact sites, which enhances phosphatidylserine import into mitochondria.
- This leads to increased mitochondrial synthesis of phosphatidylethanolamine, a major lipid prone to peroxidation and ultimately to ferroptosis.
|
| Related Techniques |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Related Applications |
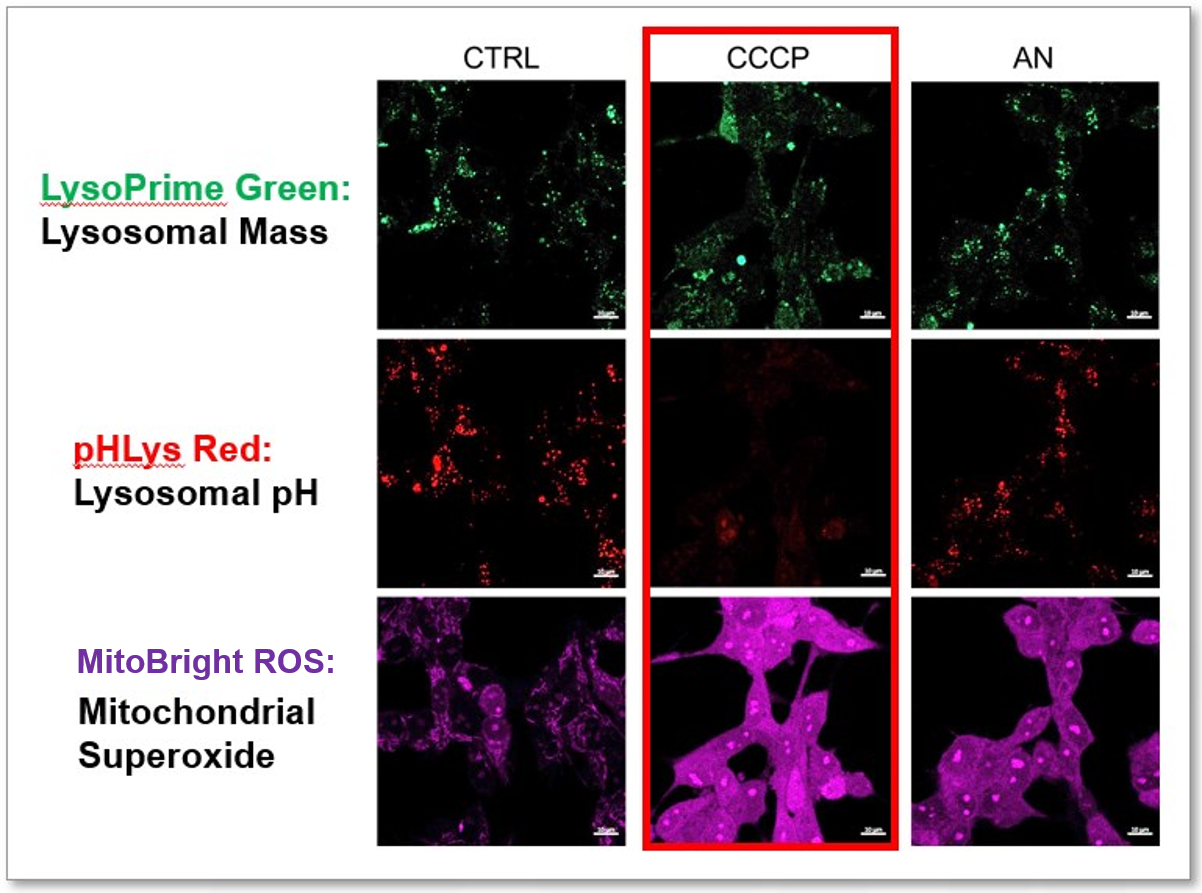
The simultaneous detection of lysosomal function with Mitochondrial ROS and intracellular Fe2+
-
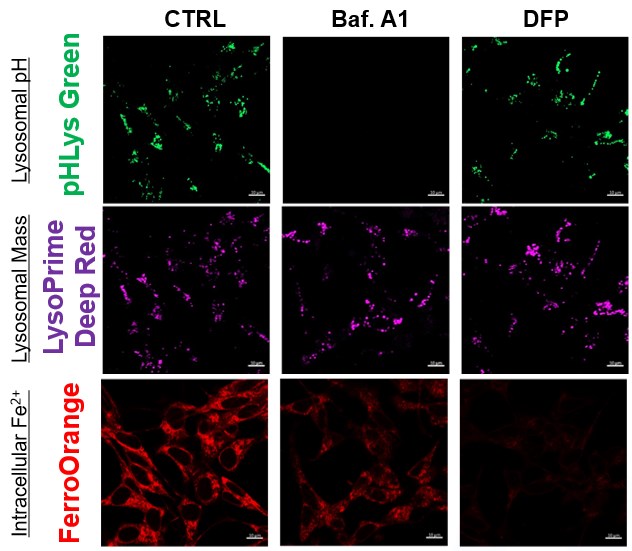
Lysosomal Function and Iron Homeostasis
-
Recent reports suggest that lysosomal neutralization can result in iron depletion, consequently leading to the disruption of cell viability. To verify this, HeLa cells were labeled with FerroOrange for Fe2+ detection, and the lysosomal mass and pH were separately detected with LysoPrime DeepRed and pHLys Green (a product currently under development). Co-staining with FerroOrange and Lysosomal dyes demonstrated that Bafilomycin A1 (Baf. A1), an inhibitor of lysosomal acidification, causes iron depletion consistent with the findings reported in the article. Interestingly, the iron chelator, Deferiprone (DFP), did not impact lysosomal pH, suggesting that lysosomal function plays a key role in managing iron homeostasis.
Reference: Ross A Weber, et. al., Mol Cell (2020)
Products in Use
- FerroOrange
- pHLys Green*
- LysoPrime Deep Red
*pHLys Green is included as a component of the "Lysosomal Acidic pH Detection Kit-Green/Deep Red".
|