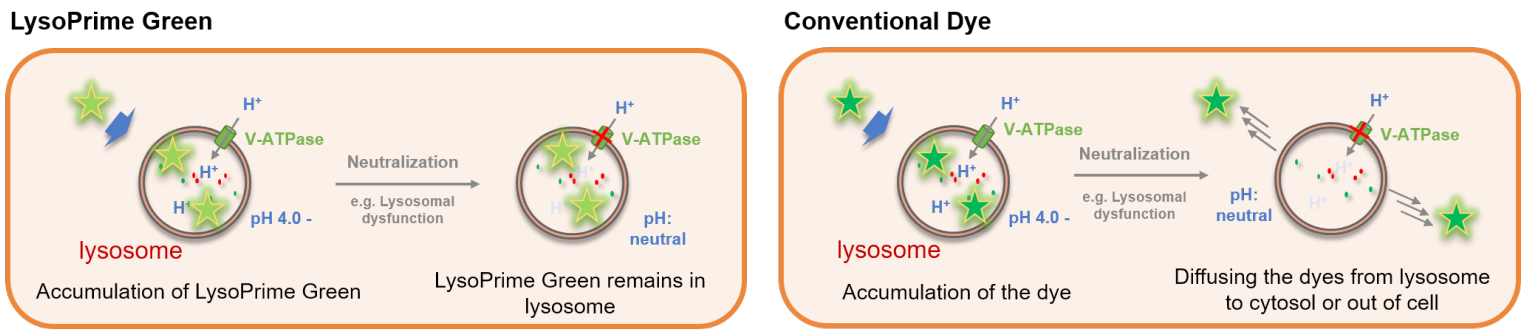
LysoPrime Green - High Specificity and pH Resistance
Lysosome Staining Dye Green
- High specificity for lysosomes, indicating accurate localization
- Resistance to pH changes in lysosomes
- Fluorescence stay up to overnight
-
Product codeL261 LysoPrime Green - High Specificity and pH Resistance
| Unit size | Price | Item Code |
|---|---|---|
| 10 μl x 1 | $146.00 | L261-10 |
| 10 μl x 3 | $303.00 | L261-12 |
<Approximate usage>
Per 10 µl: 10 plates of 35 mm dish or 10 slides of μ-Slide 8 well Chamber Slide.
<Storage Precautions>
・For long-term storage, storage below -80°C or in liquid nitrogen is recommended.
・Even store under -20°C conditions may cause degradation over time.
If storing below -80°C is difficult, please consider using the reagent as soon as possible.
・Please avoid repeated freezing and thawing, as it may cause degradation.
Description
The lysosome is an organelle in which an acid vacuole is formed by a biomembrane. Lysosomes contain various degrading enzymes and contribute to maintaining intracellular homeostasis by acting as a waste disposal system. Recent findings reveal that lysosomal dysfunction is related to some neurodegenerative disorders. Consequently, investigation of lysosomal function is attracting considerable interest in the scientific community.
Many types of small fluorescent probes are used for monitoring lysosomes in living cells. Dojindo’s LysoPrime Green overcomes known problems with fluorescent lysosome probes, such as lack of specificity for lysosomes and staining dependent on the lysosomal pH. In addition, the high-retentivity of LysoPrime Green enables long-term imaging experiments.
Lysosomal Analysis Products
| Product Name | Lysosomal pH Detection Dyes and Fluorescence Properties |
Lysosomal Quantity Detection Dyes and Fluorescence Properties |
|---|---|---|
| Lysosomal Acidic pH Detection Kit | pHLys Red Ex: 561 nm / Em: 560-650 nm |
LysoPrime Green Ex: 488 nm / Em: 500-600 nm |
| Lysosomal Acidic pH Detection Kit - Green/Deep Red | pHLys Green Ex: 488 nm / Em: 490-550 nm |
LysoPrime Deep Red Ex: 633 nm / Em: 640-700 nm |
| pHLys Red - Lysosomal Acidic pH Detection | pHLys Red Ex: 561 nm / Em: 560-650 nm |
|
| LysoPrime Deep Red - High Specificity and pH Resistance | LysoPrime Deep Red Ex: 633 nm / Em: 640-700 nm |
|
| LysoPrime Green- High Specificity and pH Resistance | LysoPrime Green Ex: 488 nm / Em: 500-600 nm |
Manual
Technical info
We compared the lysosomal localization of existing dyes and LysoPrime Green using HeLa cells expressing the lysosomal marker protein LAMP1-RFP. Staining with existing dyes resulted in a high background due to dispersion beyond lysosomes, whereas LysoPrime Green resulted in the suppressed background (fluorescent image Merged). We also measured the fluorescence intensity over the range of the fluorescence image and found that LysoPrime Green localized better to lysosomes than the existing dyes (figure below).
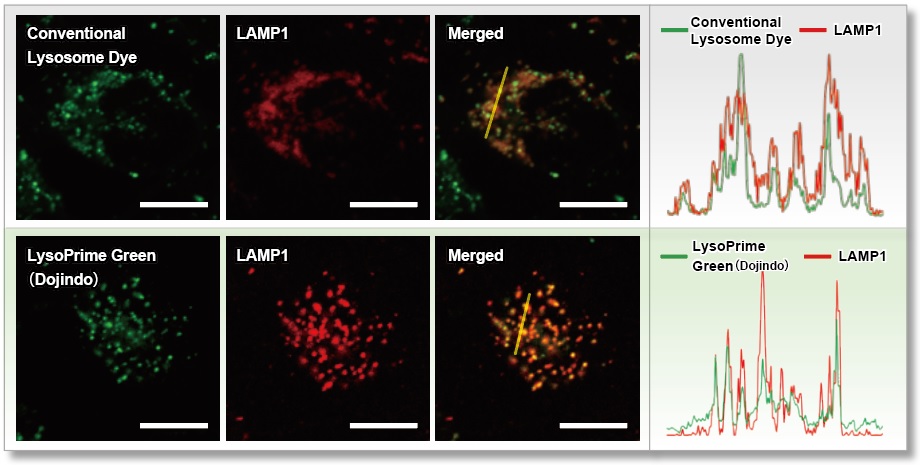
<Experimental Conditions>
Green: Ex= 488 nm, Em= 500-570 nm
Red : Ex= 561 nm, Em= 560-620 nm
Scale bar: 20 µm
Resistance to pH changes in lysosomes
LysoPrime Green and existing dyes accumulate in acidic lysosomes, but when treated with Bafilomycin A1, a lysosomal acidity inhibitor, the existing dyes leave the lysosomes when the lysosomes are changed from acidic to neutral, resulting in a significant decrease in the fluorescence signal. On the other hand, LysoPrime Green is easily retained in the lysosome, so the decrease in the fluorescence signal is suppressed and the observation results are clearer than those of existing reagents.
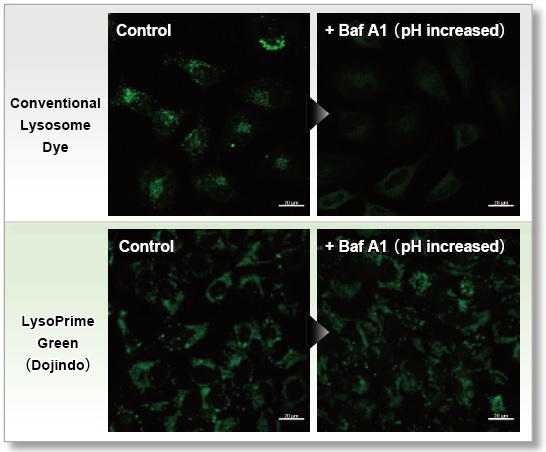
<Experimental Conditions>
Green: Ex= 488 nm, Em= 500-570 nm
Scale bar: 20 µm
High retention in lysosome
The lysosomal retention of LysoPrime Green and existing dyes were compared using cells stained with each dye. While the fluorescence of the existing dye decreased 90 minutes after staining, LysoPrime Green maintained its fluorescence intensity and showed high retention.
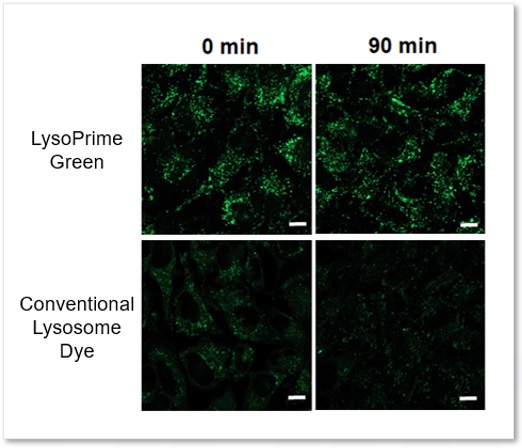
<Experimental Conditions>
Green: Ex= 488 nm, Em= 500-570 nm
Scale bar: 10 µm
In addition, HeLa cells stained with LysoPrime Green or the existing product were trypsinized and recovered, and the fluorescence intensity was checked using a flow cytometer. In contrast, LysoPrime Green showed little difference in fluorescence intensity between pre-and post-trypsin treatment staining. LysoPrime Green was found to have low leakage of dye due to pretreatment.
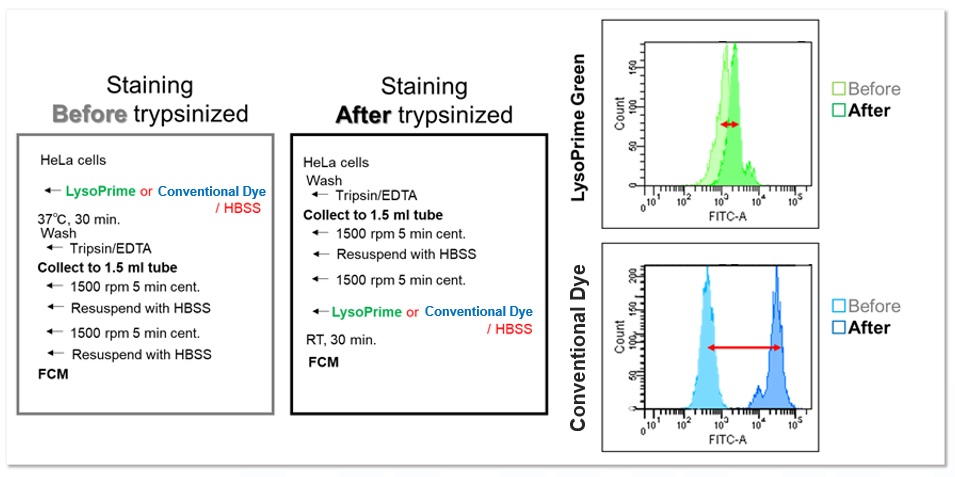
<Experimental Conditions>
Filter: FITC (Ex= 488 nm, Em= 515-545 nm)
Applications: Evaluation using lysosomal acidity inhibitors(co-staining with LysoTracker™ Red)
LysoPrime Green and LysoTracker™ Red were co-stained with LysoPrime Green and LysoTracker™ Red, respectively, and the lysosomal inhibitor Bafilomycin A1 was added for fluorescence imaging. The fluorescence signal of LysoPrime Green was almost unchanged with or without Bafilomycin A1, whereas LysoTracker™ Red showed a decrease in fluorescence signal due to lysosomal pH-neutralization caused by the addition of Bafilomycin A1. Thus, the combination of both reagents allows simultaneous evaluation of lysosome quantity and pH change. Furthermore, this fluorescence intensity could be quantified using a plate reader.
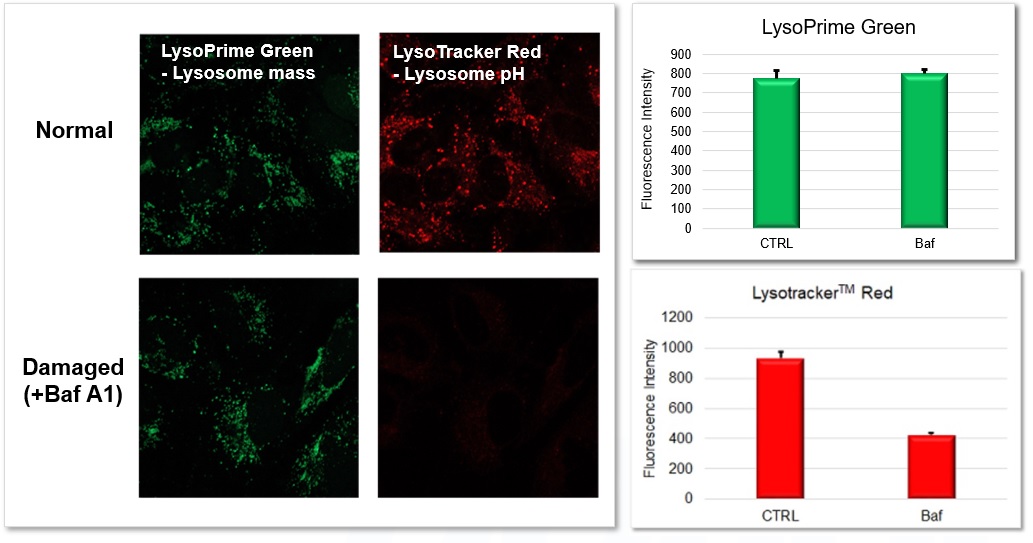
<Experimental Conditions>
LysoPrime Green: Ex=488 nm, Em=500-570 nm
LysoTracker™ Red: Ex=561 nm, Em=560-620 nm
Scale bar: 20 µm
Applications: Analysis of the localization of exosomes taken up by endocytosis in a time-dependent pathway
Fluorescence imaging was performed by adding exosomes stained with ExoSparkler Exosome Membrane Labeling Kit-Deep Red ( code: EX03) to HeLa cells stained with LysoPrime Green. The fluorescence signal of LysoPrime Green did not decrease and the temporal localization of lysosomes was confirmed, while the fluorescence signal of ExoSparkler Deep Red increased and the time-dependent increase in the amount of exosome incorporation was confirmed.
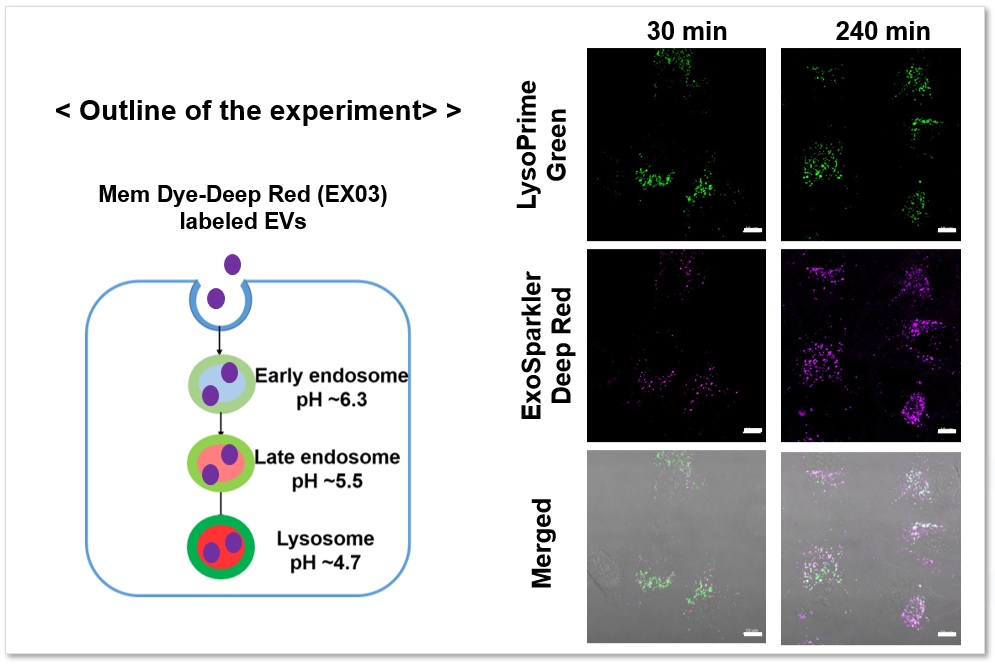
Green: Ex= 488 nm, Em= 500-570 nm
Deep Red : Ex= 640 nm, Em= 640-700 nm
Scale bar: 20 µm
The fluorescence signal of LysoPrime Green did not decrease and the localization of lysosomes over time was confirmed, while the fluorescence signal of ExoSparkler Deep Red was not observed in the group with Bafilomycin A1. The fluorescence signal of LysoPrime Green did not decrease and the localization of lysosomes over time was confirmed, while the fluorescence signal of ExoSparkler Deep Red was not observed in the group with Bafilomycin A1. This indicates that Bafilomycin A1 inhibits the maturation of endocytosis. Thus, the combination of both reagents allows for detailed analysis of the endosomal to the lysosomal pathway.
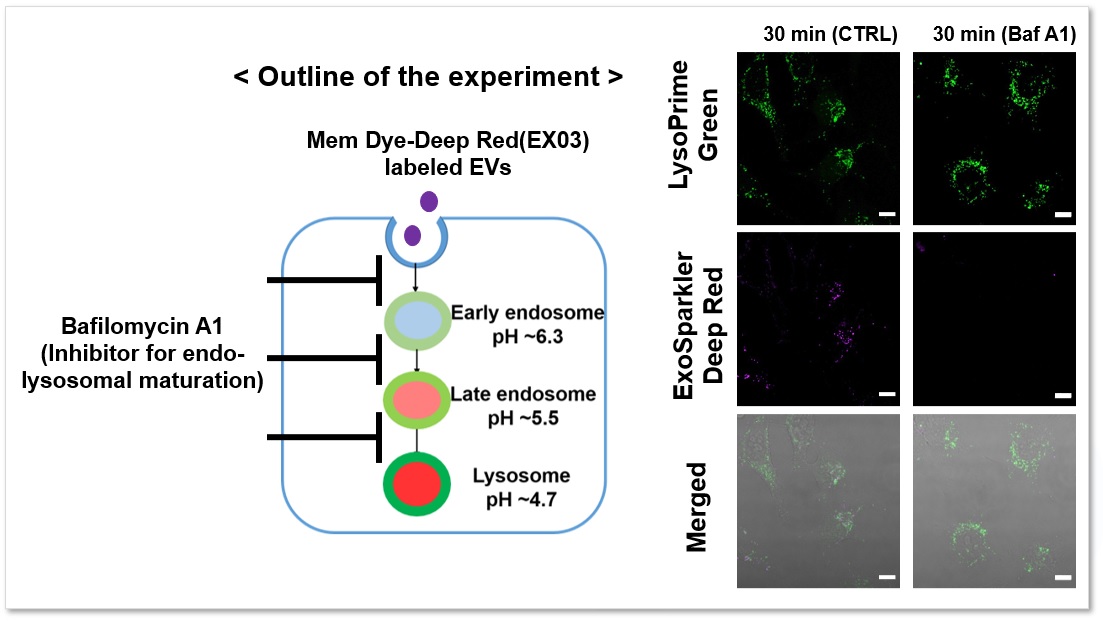
Green: Ex= 488 nm, Em= 500-570 nm
Deep Red : Ex= 640 nm, Em= 640-700 nm
Scale bar: 20 µm
Applications: Co-staining with Autophagy assay reagent
We performed fluorescence imaging by stimulating amino acid starvation of HeLa cells stained with Autophagy (Autophagosome) Detection Kit DAPRed (Code: D677) and LysoPrime Green or existing products. The fluorescence signal of the existing product decreased and the lysosomal localization could not be confirmed after starvation, whereas the fluorescence signal of LysoPrime Green did not decrease and the lysosomal localization was confirmed over time. This means that the co-localization rate of DAPRed fluorescence is higher than that of the existing products, and thus more accurate autophagy analysis can be performed.
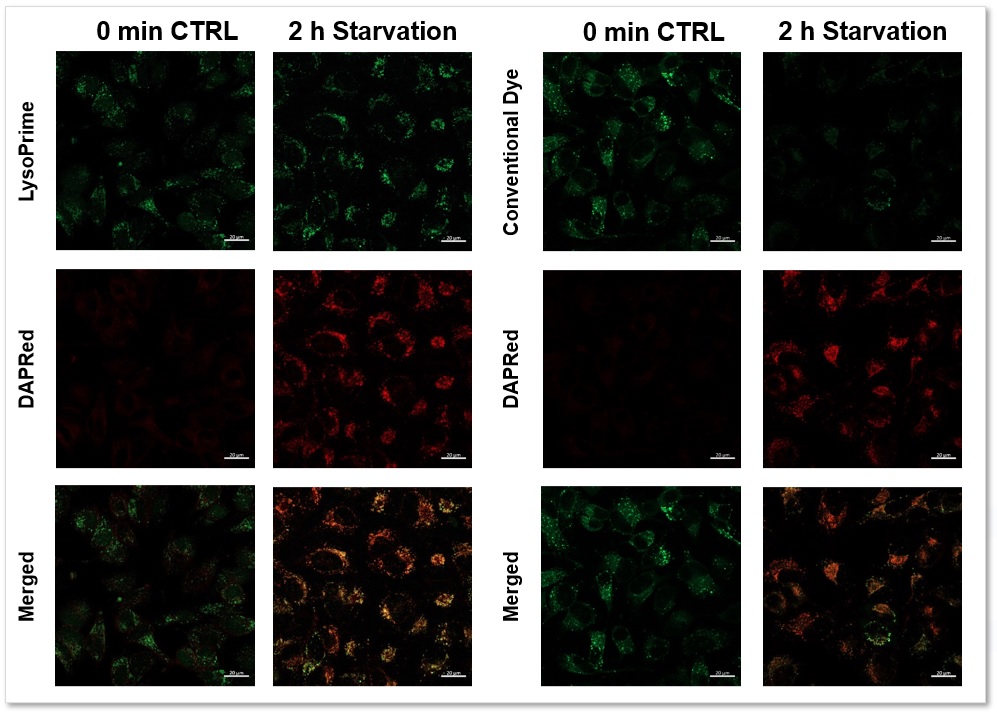
Green: Ex= 488 nm, Em= 500-570 nm
Red : Ex= 561 nm, Em= 560-650 nm
Scale bar: 20 µm
Applications: Co-staining with Mitophagy assay reagent
We performed fluorescence imaging by stimulating mitophagy induction in SHSY-5Y cells stained with Mitophagy Detection(Code: MD01) and LysoPrime Green or existing products. The fluorescence signal of LysoPrime Green did not decrease and the lysosomal localization over time was confirmed. This means that the co-localization rate of the fluorescent spots of the Mtphagy Dye is higher than that of the existing product, and thus more accurate mitophagy analysis can be performed.

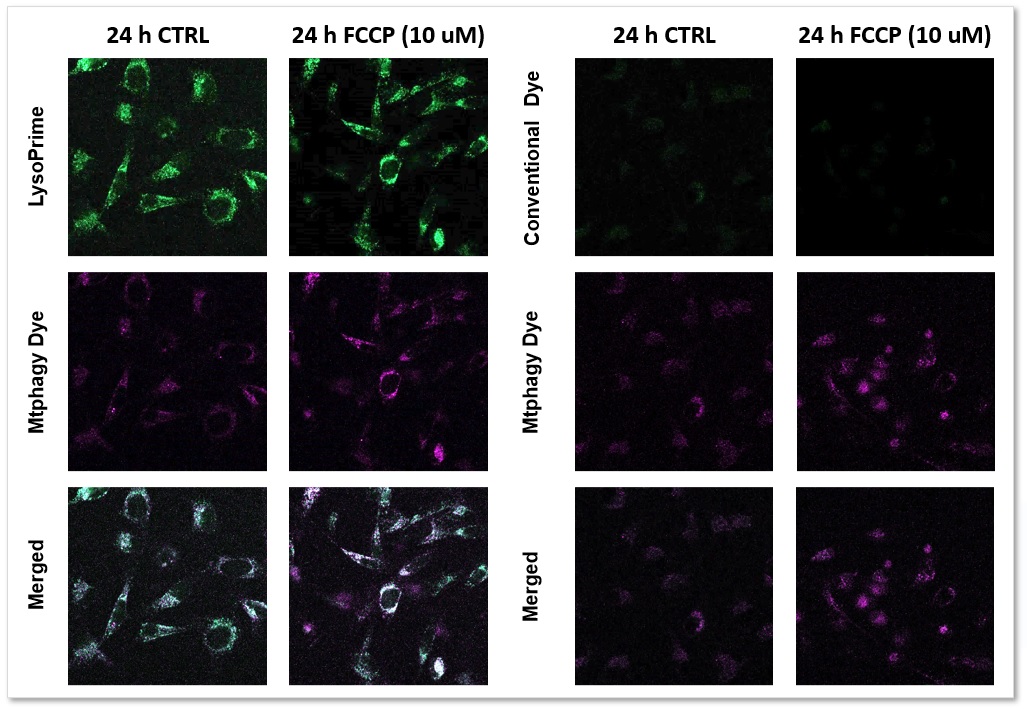
LysoPrime Green: Ex= 488 nm, Em= 500-570 nm
Mtphagy Dye: Ex= 561 nm, Em= 560-650 nm
References
| No. | Sample Type | Instrument | Reference (Link) |
|---|---|---|---|
| 1) | Cell CHO Cells |
Confocal Microscopy | M. Houser, S. Mitchell, P. Sinha, B. Lundin, O. Berezovska, M. Maesako, "Endosome and Lysosome Membrane Properties Functionally Link to γ-Secretase in Live/Intact Cells", Sensors, 2023, doi:10.3390/s23052651. |
Q & A
-
Q
Is this right that the transportation temperature and storage temperature (-80°C) are different?
-
A
No problem. There is almost no deterioration during transportation at room temperature. The storage condition is set at -80°C because the purity of the lysosomes was degraded when stored at temperatures other than -80°C for more than one month. We have confirmed that storage at room temperature (25°C) for one month does not affect the staining performance of lysosomes.
-
Q
It seems like LysoPrime Green stains other organelles besides lysosomes.
-
A
We have confirmed that the presence of excessive amounts of dye causes staining of nuclei and cytoplasm in addition to lysosomes. The optimal staining concentration is also affected by cell type and density, so be sure to check the optimal concentration before using the product for the first time. We have confirmed that working solution diluted 2,000-4,000 times with HBSS for 30 minutes can stain lysosomes without any problem.
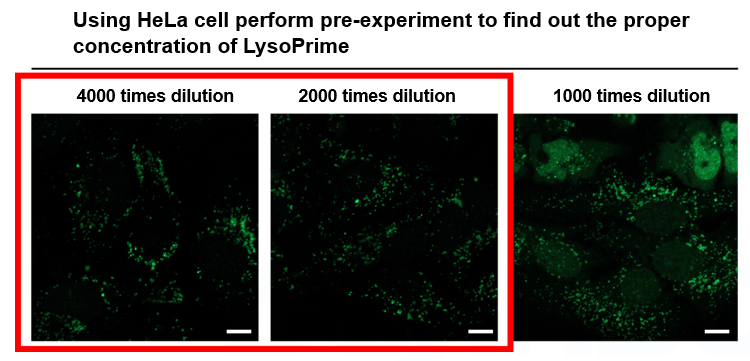
*Scale bar: 10 µm
-
Q
Is it possible to stain with serum-containing medium?
-
A
Since LysoPrime Green is affected by serum, be sure to prepare working solution in serum-free solution such as HBSS or serum-free medium.
-
Q
Should I use drug stimulation before or after staining?
-
A
LysoPrime Green accumulates in lysosomes in a pH-dependent manner and remains in the lysosomes even if the pH is changed after accumulation. On the other hand, LysoPrime Green cannot accumulate in lysosomes whose pH is changed to neutral by drug stimulation. Therefore, we recommend drug stimulation after staining to minimize the effect on staining ability.
-
Q
Can I observe with a flurescence microscope?
-
A
Yes. However, some cells may be difficult to confirm with fluorescence microscopy. Therefore, we recommend observation with confocal microscopy.
Handling and storage condition
| Storage Conditions: Freezing (-80℃) Moisture absorption attention |