|
Ferroptosis, a form of regulated cell death driven by iron-dependent lipid peroxidation, is tightly controlled by several proteins. Key regulators include GPX4, which reduces lipid hydroperoxides with glutathione (GSH) and inhibits ferroptosis, and FSP1, which provides an alternative antioxidant defense by regenerating CoQ10. Other proteins, such as iron regulatory proteins and those involved in lipid metabolism, also modulate ferroptosis by influencing iron homeostasis and lipid peroxidation pathways. Disruption or dysregulation of these protein networks can either increase susceptibility to ferroptosis or confer resistance, highlighting their critical role in maintaining cellular health.
|
-
Targeting SIRT3 sensitizes glioblastoma to ferroptosis by promoting mitophagy and inhibiting SLC7A11
Click here for the original article: Xiaohe Li, et. al., Cell Death & Disease, 2024
Point of Interest
- Sirtuin-3 (SIRT3) is a mitochondrial deacetylase that is increased in glioblastoma (GBM) brain tissue and upregulated during RAS-selective lethal 3 (RSL3)-induced GBM cell ferroptosis.
- Inhibition of SIRT3 sensitizes GBM cells to RSL3-induced ferroptosis, leading to accumulation of iron and ROS in the mitochondria, which triggers mitophagy. -Inhibition of SIRT3 also downregulates transcription of SLC7A11, which increases cellular import of cystine.
- Enforced expression of SLC7A11 in GBM cells with SIRT3 knockdown restores cellular cystine uptake and leads to cellular GSH levels.
-
Exosomal HSPB1, interacting with FUS protein, suppresses hypoxia-induced ferroptosis in pancreatic cancer by stabilizing Nrf2 mRNA and repressing P450
Click here for the original article: Lun Zhang et. al., J Cell Mol Med., 2024.
Point of Interest
- Exosomes secreted by human pancreatic cancer cells are rich in HSPB1.
- In hypoxic cells incubated with the HSPB1-containing exosomes, cell proliferation and invasion are promoted and ferroptosis is suppressed.
- Increased HSPB1 in cells leads to increased FUS expression and subsequently FUS stabilizes the mRNA of Nrf2, a known anti-ferroptosis gene.
- The exosomes administration promotes tumor growth in nude mice, which can be suppressed by knockdown of HSPB1.
-
Regulation of FSP1 myristoylation by NADPH: A novel mechanism for ferroptosis inhibition
Click here for the original article:Na Liu et. al., Redox Biology, 2024.
Point of Interest
- Exogenous NADPH not only acts as a reductant to suppress ferroptosis, but also interacts with N-myristoyltransferase 2 (NMT2).
- NMT2 modifies ferroptosis suppressor protein 1 (FSP1), a known free radical scavenging antioxidant.
- NADPH increases N-myristoylated FSP1, which localizes to the plasma membrane and provides neurons with resistance to ferroptosis.
|
|
Related Techniques
|
- Intracellular / mitochondrial ferrous ion (Fe2+) detection
- FerroOrange(intracellular), Mito-FerroGreen(mitochondrial)
|
- NAD(H) and NADP(H) redox couples assay
- NAD/NADH and NADP/NADPH Assay Kit
|
|
|
- Lipid Peroxidation Assay
- Lipid Peroxidation Probe -BDP 581/591 C11-
|
- Total ROS detection
- Highly sensitive DCFH-DA or Photo-oxidation Resistant DCFH-DA
|
- Mitochondrial superoxide detection
- MitoBright ROS Deep Red - Mitochondrial Superoxide Detection
|
- Mitochondrial membrane potential detection
- JC-1 MitoMP Detection Kit, MT-1 MitoMP Detection Kit
|
- Glutathione Quantification
- GSSG/GSH Quantification Kit
|
- Cystine Uptake detection
- Cystine Uptake Assay Kit
|
- MDA detection
- MDA Assay Kit
|
- Mitophagy or autophagy detection
- Mitophagy Detection Kit, Autophagic Flux Assay Kit
|
- Lysosomal function
- Lysosomal Acidic pH Detection Kit -Green/Red and Green/Deep Red
|
- Glycolysis/Oxidative phosphorylation Assay
-
- Glycolysis/OXPHOS Assay Kit
-
|
- Apoptosis detection in multiple samples
-
- Annexin V Apoptosis Plate Assay Kit
-
|
|
Related Applications
|
Erastin-Induced Ferroptosis: Evaluating Intracellular Uptake and Redox Balance
We investigated the transition of cellular metabolisms in A549 cells treated with erastin, a known ferroptosis inducer. Our results revealed the following.
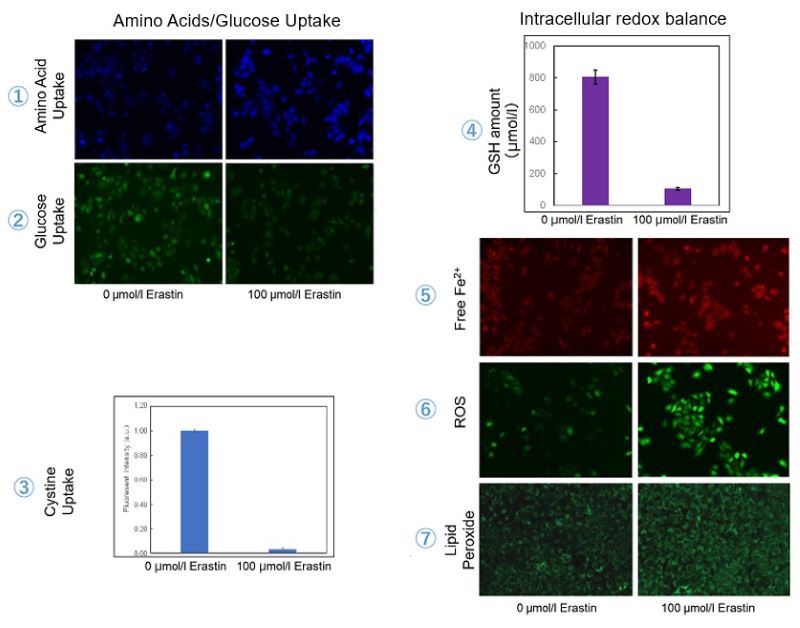
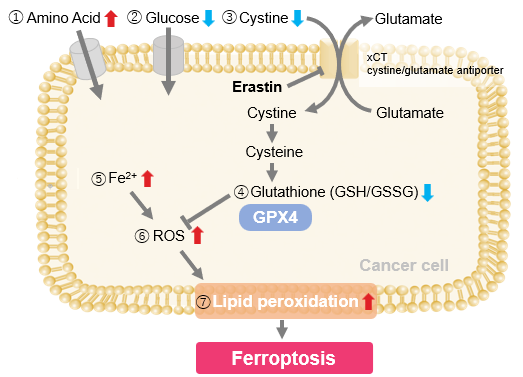
- The inhibition of cystine uptake by erastin led to a depletion of cysteine, which in turn increased the compensatory uptake of other amino acids.
- Glucose uptake, which typically promotes ferroptosis*, was found to decrease upon erastin treatment, suggesting a potential cellular self-defense mechanism.
- The depletion of cysteine resulted in a decrease in glutathione levels and an increase in Fe2+, ROS, and lipid peroxides, all of which are recognized markers of ferroptosis.
Cell Line: A549
Incubation Conditions: 100 μmol/l Erastin/MEM, 37℃, 3h
*Reference: Xinxin Song, et al., Cell Reports, (2021)
Products in Use
|

















