|
The field of ferroptosis research has grown exponentially in the past few years. This unique cell death by iron-dependent phospholipid peroxidation is regulated by multiple cellular metabolic pathways, including redox homeostasis, iron handling, mitochondrial activity, amino acid, lipid, and sugar metabolism, as well as various disease-related signaling pathways. Today, we introduce you to three highlighted articles focusing on iron resources, regulators, and the sensitive phenotype for ferroptosis in several diseases.
|
-
Microglia ferroptosis is regulated by SEC24B and contributes to neurodegeneration
Click here for the original article: Sean K. Ryan, et al., Nature Neuroscience, 26, 12-26, 2023
Point of Interest
- iPS cell-derived tri-culture system that contains microglia, neurons, and astrocytes are used in this study
- Microglia grown in a tri-culture system are highly responsive to iron and susceptible to ferroptosis
- Iron overload causes a marked shift in the microglial transcriptional state
- This microglial response contributes to neurodegeneration and is regulated by a novel ferroptosis susceptibility gene, SEC24B
-
Iron derived from autophagy-mediated ferritin degradation induces cardiomyocyte death and heart failure in mice
Click here for the original article: Jumpei Ito, et al., eLife, 10:e62174, 2021
Point of Interest
- Iron release from ferritin storage is through NCOA4-mediated autophagic degradation, known as ferritinophagy
- Deletion of Ncoa4 in mouse hearts improved cardiac function along with the attenuation of the upregulation of ferritinophagy-mediated ferritin degradation 4 weeks after pressure overload
- Free ferrous iron overload and increased lipid peroxidation were suppressed in NCOA4-deficient hearts
- Inhibition of lipid peroxidation significantly mitigated the development of pressure overload-induced dilated cardiomyopathy in wild-type mice
-
The MARCHF6 E3 ubiquitin ligase acts as an NADPH sensor for the regulation of ferroptosis
Click here for the original article: Kha The Nguyen, et al., Nature Cell Biology, 24, 1239-1251, 2022
Point of Interest
- The level of the anabolic reductant NADPH is a biomarker of ferroptosis sensitivity
- The transmembrane endoplasmic reticulum MARCHF6 E3 ubiquitin ligase recognizes NADPH through its C-terminal regulatory region
- This interaction upregulates the E3 ligase activity of MARCHF6, thus downregulating ferroptosis
- Inhibiting ferroptosis rescued the growth of MARCHF6-deficient tumours and peri-natal lethality of Marchf6–/– mice.
|
|
Related Techniques
|
- Intracellular / mitochondrial ferrous ion (Fe2+) detection
- FerroOrange(intracellular), Mito-FerroGreen(mitochondrial)
|
|
|
- Lipid Peroxidation Assay
- Lipid Peroxidation Probe -BDP 581/591 C11-
|
- Total ROS detection
- Highly sensitive DCFH-DA or Photo-oxidation Resistant DCFH-DA
|
- Mitochondrial superoxide detection
- MitoBright ROS Deep Red - Mitochondrial Superoxide Detection
|
- Mitochondrial membrane potential detection
- JC-1 MitoMP Detection Kit, MT-1 MitoMP Detection Kit
|
- Glutathione Quantification
- GSSG/GSH Quantification Kit
|
- Cystine Uptake detection
- Cystine Uptake Assay Kit
|
- MDA detection
- MDA Assay Kit
|
- Mitophagy or autophagy detection
- Mitophagy Detection Kit, Autophagic Flux Assay Kit
|
- Lysosomal function
- Lysosomal Acidic pH Detection Kit -Green/Red and Green/Deep Red
|
- Glycolysis/Oxidative phosphorylation Assay
-
- Glycolysis/OXPHOS Assay Kit
-
|
- Apoptosis detection in multiple samples
-
- Annexin V Apoptosis Plate Assay Kit
-
|
|
Related Applications
|
Erastin-Induced Ferroptosis: Evaluating Intracellular Uptake and Redox Balance
We investigated the transition of cellular metabolisms in A549 cells treated with erastin, a known ferroptosis inducer. Our results revealed the following.
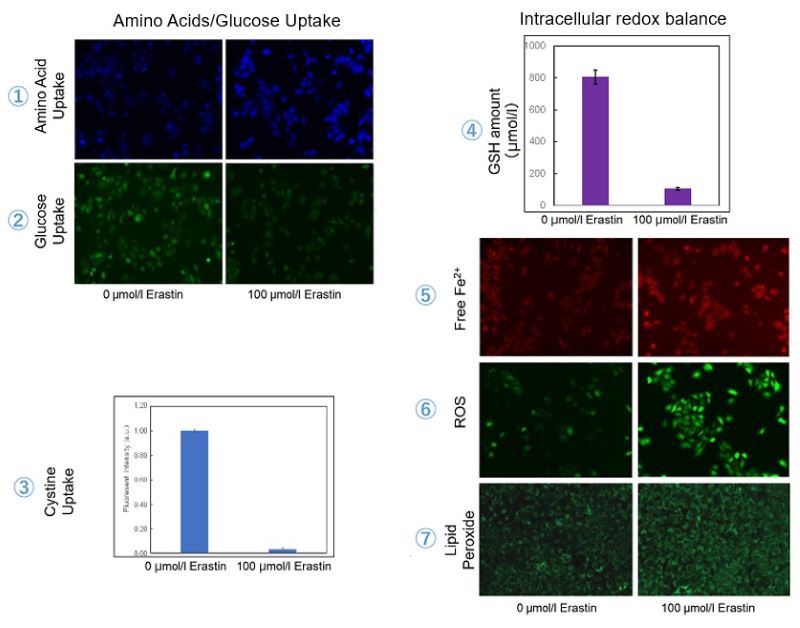
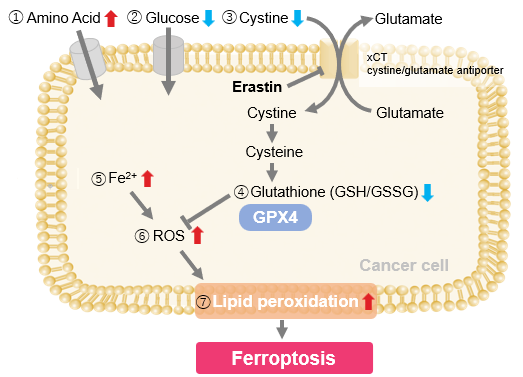
- The inhibition of cystine uptake by erastin led to a depletion of cysteine, which in turn increased the compensatory uptake of other amino acids.
- Glucose uptake, which typically promotes ferroptosis*, was found to decrease upon erastin treatment, suggesting a potential cellular self-defense mechanism.
- The depletion of cysteine resulted in a decrease in glutathione levels and an increase in Fe2+, ROS, and lipid peroxides, all of which are recognized markers of ferroptosis.
Cell Line: A549
Incubation Conditions: 100 μmol/l Erastin/MEM, 37℃, 3h
*Reference: Xinxin Song, et al., Cell Reports, (2021)
Products in Use
|

















