|
Scientists have unveiled that the selective autophagy adaptor NCOA4, which targets ferritin (a process known as ferritinophagy), is upregulated in pancreatic cancer. This upregulation ensures the availability of iron, thus encouraging tumor progression. Fascinatingly, ferritinophagy facilitates the synthesis of iron–sulfur cluster proteins, which help maintain mitochondrial homeostasis. Discover how the authors employed separate iron-detecting probes: FerroOrange for the cytosol and Mito-FerroGreen for the mitochondria (refer to Figure 1I and Supplemental Figure S4J).
|
|
NCOA4-Mediated Ferritinophagy Is a Pancreatic Cancer Dependency via Maintenance of Iron Bioavailability for Iron–Sulfur Cluster Proteins
Click here for the original article: Naiara Santana-Coodina, et. al., Cancer Discovery (2022)
Point of Interest
- - Ferritinophagy, the process by which iron is released from storage for use in cells, is found to be upregulated in pancreatic ductal adenocarcinoma (PDAC), promoting tumor growth.
- - It's shown to support the synthesis of iron–sulfur cluster proteins, thereby maintaining mitochondrial stability.
- - Blocking the ferritinophagy adapter NCOA4 delays tumor growth and extends survival, although compensatory iron-gathering pathways may develop.
|
| Related Techniques |
|
|
|
|
|
|
|
|
|
|
|
|
| Related Applications |
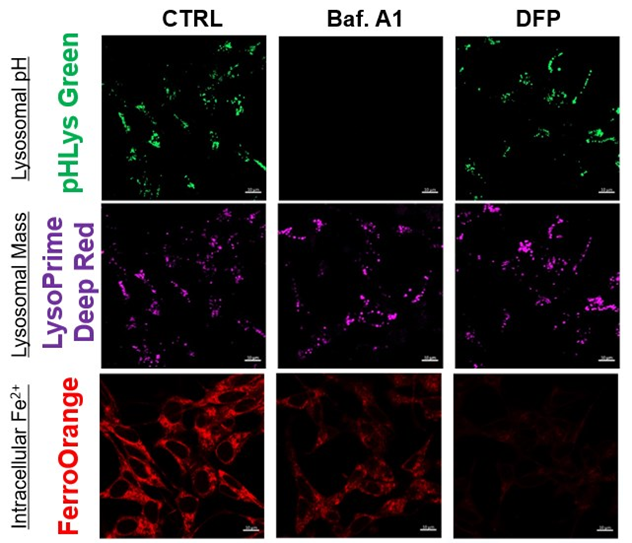
The simultaneous detection of lysosomal function with Mitochondrial ROS and intracellular Fe2+
Lysosomal Function and Iron Homeostasis
-
Recent reports suggest that lysosomal neutralization can result in iron depletion, consequently leading to the disruption of cell viability. To verify this, HeLa cells were labeled with FerroOrange for Fe2+ detection, and the lysosomal mass and pH were separately detected with LysoPrime DeepRed and pHLys Green (a product currently under development). Co-staining with FerroOrange and Lysosomal dyes demonstrated that Bafilomycin A1 (Baf. A1), an inhibitor of lysosomal acidification, causes iron depletion consistent with the findings reported in the article. Interestingly, the iron chelator, Deferiprone (DFP), did not impact lysosomal pH, suggesting that lysosomal function plays a key role in managing iron homeostasis.
Reference: Ross A Weber, et. al., Mol Cell (2020)
Products in Use
- FerroOrange
- pHLys Green*
- LysoPrime Deep Red
*pHLys Green is included as a component of the "Lysosomal Acidic pH Detection Kit-Green/Deep Red".
|
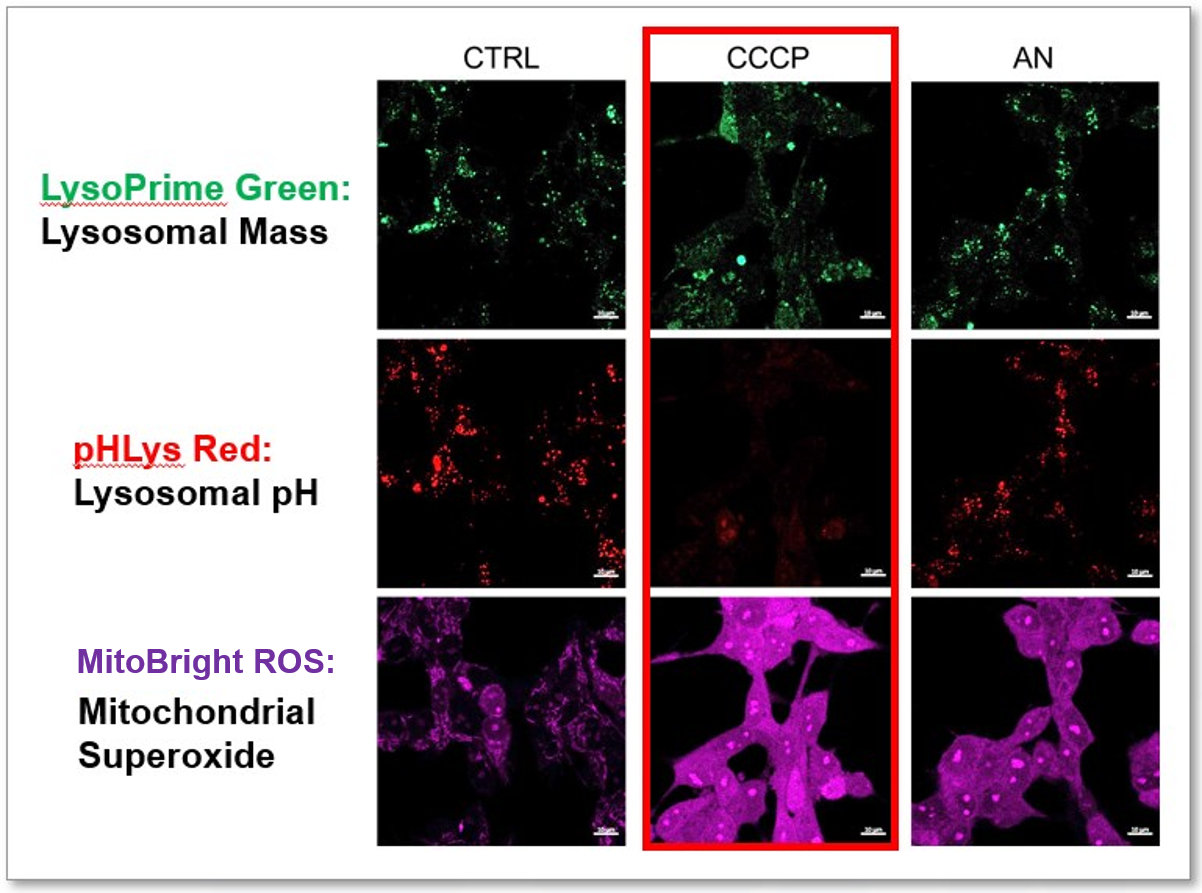
Lysosomal Function and Mitochondrial ROS
-
CCCP and Antimycin are recognized inducers of mitochondrial ROS, linked to the loss of mitochondrial membrane potential. Recent studies have shown that CCCP induces not only mitochondrial ROS but also lysosomal dysfunction. To observe mitochondrial ROS, HeLa cells were labeled with MitoBright ROS Deep Red for Mitochondrial Superoxide Detection, and the lysosomal mass and pH were independently detected with LysoPrime Green and pHLys Red. Co-staining with MitoBright ROS and Lysosomal dyes revealed that CCCP, unlike Antimycin, triggers concurrent lysosomal neutralization and mitochondrial ROS induction.
Reference: Benjamin S Padman, et. al., Autophagy (2013)
Products in Use
- LysoPrime Green
- pHLys Red
- Lysosomal Acidic pH Detection Kit
- MitoBright ROS - Mitochondrial Superoxide Detection
|

















