Science Note: Cellular metabolism
Why Targeting Tumor Metabolism is attractive? [Jan. 21, 2025]
Open / Close the Article
Targeting metabolic heterogeneity in the tumor microenvironment (TME) is critical in cancer research because metabolic diversity affects cancer and immune cell function, promoting tumor progression and immune suppression. In addition to previous findings, recent studies have identified acetyl-CoA carboxylase and glycosphingolipid synthesis as key mediators of immune evasion in the TME. In this note, we introduce a review of cancer-promoting mechanisms driven by metabolic heterogeneity in the TME, along with recent findings.
|
|||
| Metabolic programming and immune suppression in the tumor microenvironment Click here for the original article: Emily N. Arner, et. al., Cancer Cell, 2023. |
Acetyl-CoA carboxylase obstructs CD8+ T cell lipid utilization in the tumor microenvironment Click here for the original article: Elizabeth G. Hunt, et. al., Cell Metabolism, 2024. |
Glycosphingolipid synthesis mediates immune evasion in KRAS-driven cancer Click here for the original article: Mariluz Soula, et. al., Nature, 2024. |
|
|
Point of Interest - Altered nutrients and signals in the TME suppress effector immune cells and favor regulatory immune cells, contributing to immune suppression. - Targeting metabolic heterogeneity in the TME is a promising strategy to overcome immune suppression and improve the efficacy of cancer immunotherapy.
|
Point of Interest - The solid TME activates acetyl-coenzyme A carboxylase (ACC) in T cells, causing lipid droplet accumulation in tumor-infiltrating T cells, which inhibits FAO. - Restricting ACC activity rewires T cell metabolism to maintain energy during TME stress. |
Point of Interest - Blocking glycosphingolipid synthesis increases IFNGR1 levels and enhances IFNγ-induced growth arrest and pro-inflammatory signaling. - Inhibiting glycosphingolipid production increases natural killer and CD8+ T cell activity and synergizes with checkpoint blockade therapy to enhance anti-tumor responses. |
|
| Related Techniques | |||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | ||
| Lactic Acid Measurement | Lactate Assay Kit-WST | ||
| Oxygen Consumption Rate(OCR) Detection | Extracellular OCR Plate Assay Kit | ||
| Mitochondrial membrane potential detection | JC-1 MitoMP Detection Kit, MT-1 MitoMP Detection Kit | ||
| Glucose Uptake Capacity Assay | Glucose Uptake Assay Kit-Blue / Green / Red | ||
| Fatty Acid Uptake Capacity Assay | Fatty Acid Uptake Assay Kit | ||
| Lipid Droplet Staining | Lipi-Blue/ Green/ Red/ Deep Red | ||
| Lipid Droplet Detection Kit | Lipid Droplet Assay Kit - Blue / Deep Red | ||
| Apoptosis detection in multiple samples | Annexin V Apoptosis Plate Assay Kit | ||
| Cell proliferation/ cytotoxicity assay | Cell Counting Kit-8 and Cytotoxicity LDH Assay Kit-WST | ||
| Related Applications | |||
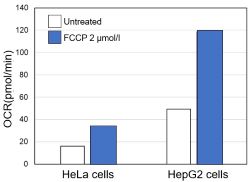
Comparison of metabolic pathway in two types of cancer cellsThe dependence of OXPHOS and Glycolysis in two types of cancer cells, HeLa and HepG2, were compared based on Lactate production, ATP levels, and OCR values. Many cancer cells produce ATP through the glycolytic pathway. On the other hand, it has been recently reported that cancer cells whose glycolytic pathway is suppressed survive by shifting their energy metabolism to OXPHOS by enhancing mitochondrial function, and the dependency of metabolic pathways differs depending on cell lines. |
|||
<Evaluation by Lactate production and ATP levels>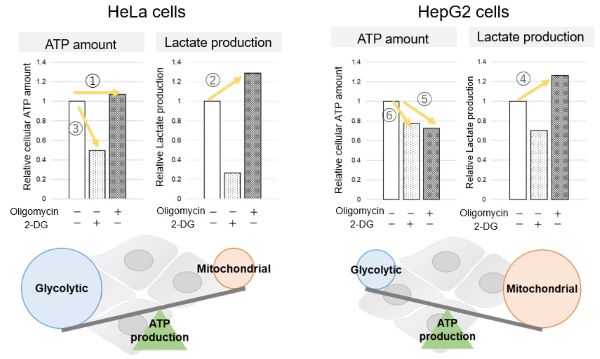
We confirmed the changes in ATP and Lactate production when ATP synthesis by OXPHOS was inhibited by Oligomycin stimulation and by 2-Deoxy-D-glucose (2-DG) in the glycolytic pathway. The results showed that HeLa cells depend on Glycolysis and HepG2 cells depend on OXPHOS to synthesize ATP. |
<Evaluation by Lactate production and ATP levels> Products in Use
|
||
<Evaluation by OCR value> |
Using the same number of cells, we measured the OCR value when cellular oxygen consumption was promoted by stimulating the cells with FCCP, a mitochondrial uncoupling agent. The results showed that HepG2 cells had higher OCR values than HeLa cells, suggesting a greater dependence on OXPHOS, correlating with the results obtained from ATP level and Lactate production. Products in Use |
||
How Lipid Droplets and Lipid Peroxides Affect Neuronal Toxicity [Nov. 5, 2024]
Open / Close the Article
In recent neurodegenerative disease research has shown that lipid metabolism, such as lipid droplets and lipid peroxides, influences neuronal damage. Here are some of the papers that have identified a relationship between lipid metabolism and the neurotoxic environment.Neuronal toxicity in neurodegenerative diseases is often associated with the accumulation of lipid droplets and lipid peroxides, which are by-products of lipid metabolism. Lipid droplets in neurons and glia serve as storage for lipids, but excessive accumulation can impair cellular function. Lipid peroxides resulting from oxidative stress are particularly damaging, generating reactive oxygen species that lead to cellular dysfunction and cell death. In tauopathies*, lipid peroxides are transferred to glial cells, exacerbating the inflammation and oxidative stress that contribute to the neurotoxic environment. *Tauopathy is a neurodegenerative disease characterized by abnormal accumulation of tau protein. |
|||
| APOE4/4 is linked to damaging lipid droplets in Alzheimer’s disease microglia Click here for the original article: Michael S. Haney, et. al., Nature, 2024. |
Tau is required for glial lipid droplet formation and resistance to neuronal oxidative stress Click here for the original article: Lindsey D. Goodman, et. al., Nature Neuroscience, 2024. |
Microglial lipid droplet accumulation in tauopathy brain is regulated by neuronal AMPK Click here for the original article: Yajuan Li, et. al., Cell Metabolism, 2024. |
|
|
Point of Interest - Fibrillar Aβ induces ACSL1 expression, triglyceride synthesis and lipid droplet accumulation in APOE-dependent microglia. - Conditioned media from lipid droplet-containing microglia lead to tau phosphorylation and neurotoxicity, linking genetic risk factors for AD to potential therapeutic targets.
|
Point of Interest - Glial endogenous tau is essential for lipid droplet formation and protection against toxic neuronal lipids in flies, rats and human cells. - Flies lacking glial endogenous tau show motor defects and reduced lifespan, which can be alleviated by antioxidants such as N-acetylcysteine amide. |
Point of Interest - Unsaturated lipid transfer from tauopathy neurons to microglia leads to lipid droplet accumulation, oxidative stress, inflammation and impaired phagocytosis. - AMPK inhibits neuronal lipogenesis and supports lipophagy, reducing lipid transfer to microglia, whereas AMPK depletion exacerbates lipid accumulation and neuropathology. |
|
| Related Techniques | |||
| Lipid Droplet Staining | Lipi-Blue/ Green/ Red/ Deep Red | ||
| Lipid Droplet Detection Kit | Lipid Droplet Assay Kit - Blue / Deep Red | ||
| Intracellular / mitochondrial lipid peroxidation detection | Liperfluo, MitoPeDPP | ||
| Total ROS detection | Highly sensitive DCFH-DA or Photo-oxidation Resistant DCFH-DA | ||
| Mitochondrial superoxide detection | MitoBright ROS Deep Red - Mitochondrial Superoxide Detection | ||
| Glutathione Quantification | GSSG/GSH Quantification Kit | ||
| First-time autophagy research | Autophagic Flux Assay Kit | ||
| Lysosomal function | Lysosomal Acidic pH Detection Kit -Green/Red and Green/Deep Red | ||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | ||
| Apoptosis detection in multiple samples | Annexin V Apoptosis Plate Assay Kit | ||
| Cell proliferation/ cytotoxicity assay | Cell Counting Kit-8 and Cytotoxicity LDH Assay Kit-WST | ||
| Related Applications | |||
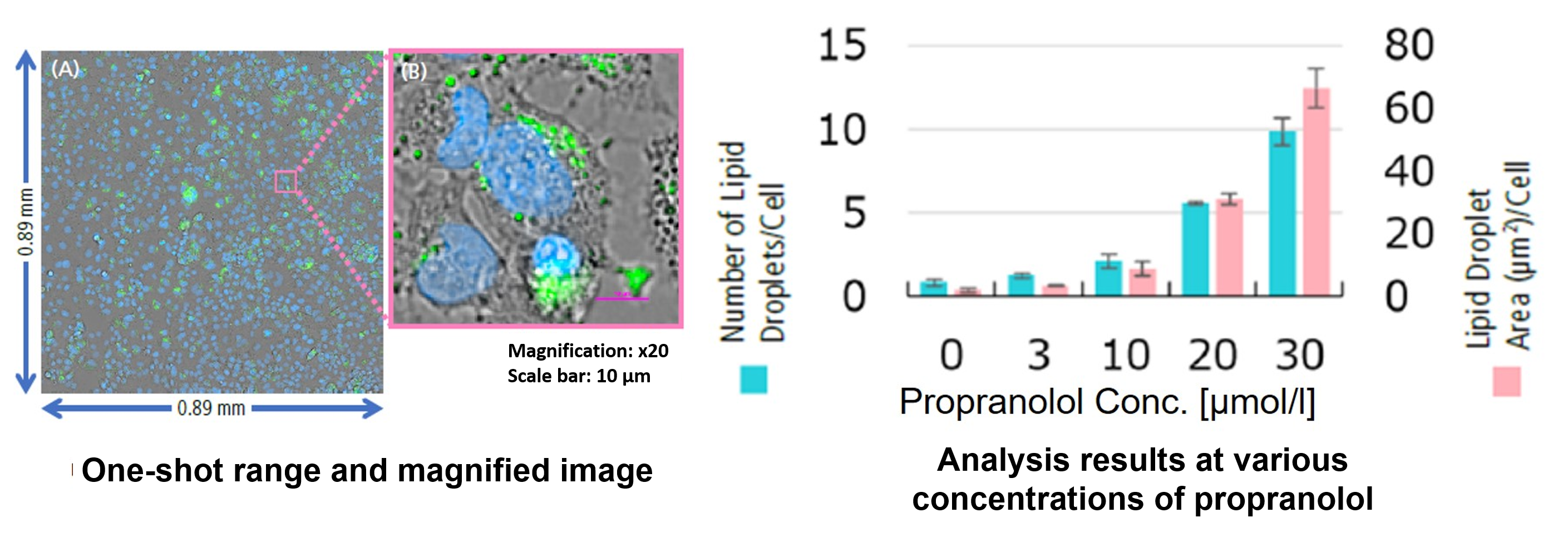
Hepatotoxicity test of drug-induced lipidosis using high-content imagingPropranolol (a sympathetic β-receptor blocker) was added to a human hepatocellular carcinoma cell line (HepG2 cells), and changes in lipid droplets were observed under a fluorescence microscope. The accumulation of lipid droplets was analyzed by measuring the number, area, and fluorescence intensity of lipid droplets from the acquired microscopic images. |
|||
<Lipid droplet imaging data>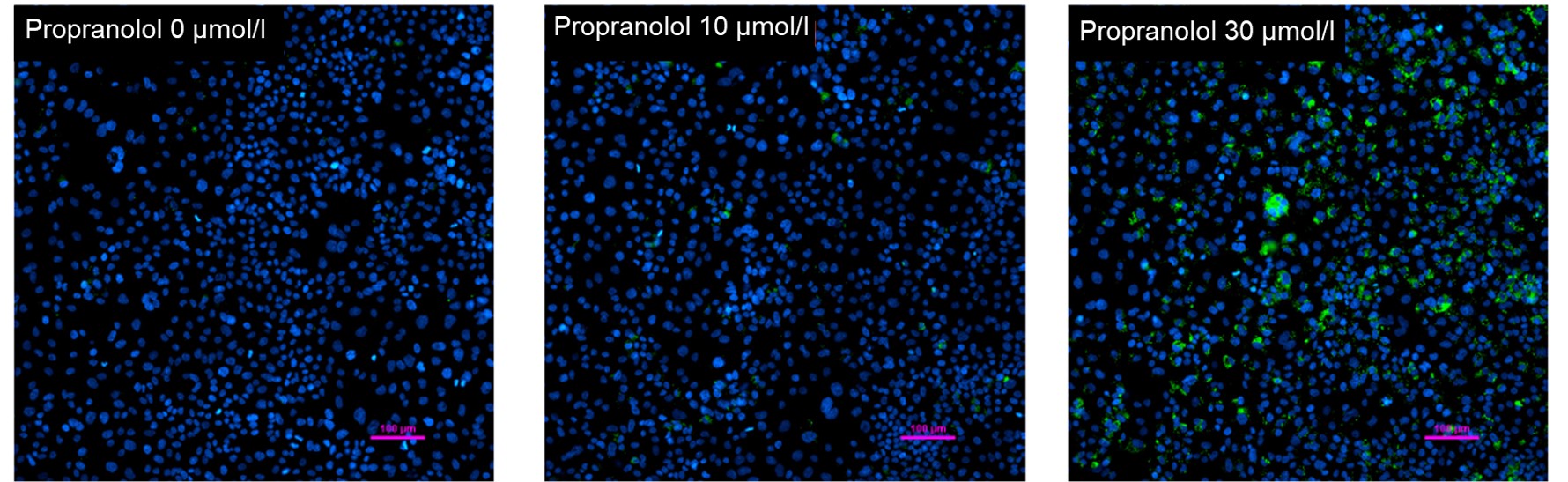
Nucleus (blue: Hoechst 33342 ): Ex 385 nm, Em 460 nm |
HepG2 cells were treated with propranolol 0, 10, or 30 μmol/l, lipid droplets were stained with Lipi-Green and nuclei with Hoechst 33342 and observed using a fluorescence microscope (Ti2-E inverted microscope). The results showed that lipid droplets increased in a propranolol concentration-dependent manner.
Related Products |
||
<Analysis of lipid droplet accumulation relative to drug treatment concentration> High Content Analysis (HCA) microscope system For details of staining and analysis methods, please refer to "APPLICATION NOTE: Hepatotoxicity test of drug-induced lipidosis using high-content imaging" by Nikon Corporation. |
From the fluorescence images obtained, the accumulation of lipid droplets per cell was analyzed by measuring cell number from nuclei and area, number, and fluorescence intensity from lipid droplets. The results showed that the number and area of lipid droplets increased in a propranolol concentration-dependent manner, with lipid droplets forming significantly under concentration conditions of 20 μmol/l or higher. The DS-Qi2 camera, which can capture a wide range of cellular areas in a single shot, was used for imaging, and the EDF module of NIS-Elements software, which can acquire focused images of all lipid droplets, was used for analysis, enabling quantitative analysis with highly reliable statistical data. The EDF module of the NIS-Elements software allows for the acquisition of focused images of all lipid droplets. |
||
Regulation of Glycolytic Activity for Neuron Maintenance [Sep. 17, 2024]
Open / Close the Article
Recent research on neurodegenerative diseases is revealing that although neurons require glycolysis, excessive neuronal glycolysis causes cognitive decline. Here are some of the papers showing glycolytic activity in neurons and neurodegenerative diseases.Neurons require considerable energy to function, and while they rely primarily on glucose, they have a relatively low rate of glycolysis compared to other cells. This limited glycolytic activity is essential for maintaining mitochondrial health and preventing redox stress. Neurons use glucose-derived metabolites from glycolysis to fuel the tricarboxylic acid (TCA) cycle for ATP production. Disruptions in glycolysis can impair cognitive function and lead to neurodegenerative diseases, highlighting the balance that neurons maintain between glycolysis and mitochondrial energy production. |
|||
| Weak neuronal glycolysis sustains cognition and organismal fitness Click here for the original article: Daniel Jimenez-Blasco, et. al., Nature Metabolism, 2024. |
Restoring hippocampal glucose metabolism rescues cognition across Alzheimer’s disease pathologies Click here for the original article: Paras S. Minhas, et. al., Science, 2024. |
Neurons require glucose uptake and glycolysis in vivo Click here for the original article: Huihui Li, et. al., Cell Reports, 2023. |
|
|
Point of Interest - Increasing glycolysis in neurons causes mitochondrial abnormalities, redox stress, bioenergetic deficiencies and cognitive decline in mice. - Restoring brain NAD+ or reducing mitochondrial stress reverses cognitive decline caused by excessive neuronal glycolysis.
|
Point of Interest - Blocking IDO1 improves glucose metabolism and lactate production in the hippocampus and improves neuronal function in AD mice. - IDO1 inhibition, originally developed for cancer, may treat neurodegenerative diseases such as Alzheimer's and Parkinson's by restoring brain metabolism. |
Point of Interest - Deletion of GLUT3 or PKM1 in neurons results in age-related learning deficits, altered glucose metabolism, and changes in brain volume. - Neurons require glycolysis for normal function, with compensatory mitochondrial changes occurring when glycolysis is disrupted. |
|
| Related Techniques | |||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | ||
| Mttochondrial membrane potential detection | JC-1 MitoMP Detection Kit, MT-1 MitoMP Detection Kit | ||
| Mitochondrial superoxide detection | MitoBright ROS Deep Red - Mitochondrial Superoxide Detection | ||
| Oxygen consumption rate assay | Extracellular OCR Plate Assay Kit | ||
| NAD(H) and NADP(H) redox couples assay | NAD/NADH and NADP/NADPH Assay Kit | ||
| First-time autophagy research | Autophagic Flux Assay Kit | ||
| Glutathione Quantification | GSSG/GSH Quantification Kit | ||
| Glucose Metabolism Assay | Glucose Assay Kit-WST | ||
| ADP/ATP Ratio Assay | ADP/ATP Ratio Assay Kit-Luminescence | ||
| Related Applications | |||
Comparison of metabolic pathway in two types of cancer cellsThe dependence of OXPHOS and Glycolysis in two types of cancer cells, HeLa and HepG2, were compared based on Lactate production, ATP levels, and OCR values. Many cancer cells produce ATP through the glycolytic pathway. On the other hand, it has been recently reported that cancer cells whose glycolytic pathway is suppressed survive by shifting their energy metabolism to OXPHOS by enhancing mitochondrial function, and the dependency of metabolic pathways differs depending on cell lines. |
|||
<Evaluation by Lactate production and ATP levels>
We confirmed the changes in ATP and Lactate production when ATP synthesis by OXPHOS was inhibited by Oligomycin stimulation and by 2-Deoxy-D-glucose (2-DG) in the glycolytic pathway. The results showed that HeLa cells depend on Glycolysis and HepG2 cells depend on OXPHOS to synthesize ATP. |
<Evaluation by Lactate production and ATP levels> Products in Use |
||
<Evaluation by OCR value> |
Using the same number of cells, we measured the OCR value when cellular oxygen consumption was promoted by stimulating the cells with FCCP, a mitochondrial uncoupling agent. The results showed that HepG2 cells had higher OCR values than HeLa cells, suggesting a greater dependence on OXPHOS, correlating with the results obtained from ATP level and Lactate production. Products in Use |
||
Metabolic Alterations Promoting Cancer, Neurodegeneration and Senescence [Aug. 6, 2024]
Open / Close the Article
|
Metabolic alterations play a critical role in the development and progression of diseases such as cancer, neurodegeneration and senescence. In cancer, altered metabolism supports rapid cell growth and survival, often through the Warburg effect, where cancer cells rely heavily on glycolysis even in the presence of oxygen. Neurodegenerative diseases such as Alzheimer's and Parkinson's are associated with dysregulated energy metabolism leading to impaired neuronal function and increased oxidative stress. Senescence, the process of cellular aging, involves metabolic reprogramming that contributes to the cessation of cell division and the secretion of pro-inflammatory factors, affecting tissue homeostasis and promoting age-related diseases. |
|||
| Targeting PHGDH reverses the immunosuppressive phenotype of tumor-associated macrophages through α-ketoglutarate and mTORC1 signaling Click here for the original article: Zhengnan Cai, et. al., Cellular & Molecular Immunology, 2024. |
LXR/CD38 activation drives cholesterol-induced macrophage senescence and neurodegeneration via NAD+ depletion Click here for the original article: Ryo Terao, et. al., Cell Reports, 2024. |
TREM1 disrupts myeloid bioenergetics and cognitive function in aging and Alzheimer’s disease mouse models Click here for the original article: Edward N. Wilson, et. al., Nat Neurosci., 2024. |
|
|
Point of Interest - PHGDH-mediated serine biosynthesis activates mTORC1 signaling and maintains M2-like macrophages. - Loss of PHGDH disrupts metabolism and shifts M2-like tumor-associated macrophages to an M1-like phenotype, suggesting that PHGDH inhibition may suppress tumor progression. |
Point of Interest - Cholesterol-mediated NAD+ depletion induces macrophage senescence, contributing to age-related macular degeneration (AMD) and neurodegeneration. - NAD+ augmentation and senolytics reverse macrophage senescence, preventing AMD and neurodegeneration. |
Point of Interest - The age-related increase in TREM1 suppresses glucose metabolism, particularly the production of ribose-5P, which is essential for both glycolysis and the biosynthesis of purines, pyrimidines and NAD+, leading to disruption of homeostatic microglial metabolism and immune function. - TREM1 deficiency prevents age-related changes in myeloid metabolism, inflammation and hippocampal memory function. |
|
| Related Techniques | |||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | ||
| Mitochondrial function/glycolysis detection | Glycolysis/JC-1 MitoMP Assay Kit | ||
| Oxygen consumption rate assay | Extracellular OCR Plate Assay Kit | ||
| NAD(H) and NADP(H) redox couples assay | NAD/NADH and NADP/NADPH Assay Kit | ||
| Cellular senescence detection | SPiDER-βGal for live-cell imaging or flow cytometry / microplate reader / tissue samples | ||
| Lysosomal function | Acidic pH Detection Kit-Green/Red and Green/Deep Red | ||
| Endocytosis Detection detection | ECGreen-Endocytosis Detection | ||
| Mitochondria Detection | Mitophagy Detection Kit, MitoBright ROS Deep Red | ||
| Mitochondrial superoxide detection | MitoBright ROS Deep Red - Mitochondrial Superoxide Detection | ||
| Cell Proliferation / Cytotoxicity Assay | Cell Counting Kit-8 and Cytotoxicity LDH Assay Kit-WST | ||
| Related Applications | |||
Comparison of metabolic pathway in two types of cancer cellsThe dependence of OXPHOS and Glycolysis in two types of cancer cells, HeLa and HepG2, were compared based on Lactate production, ATP levels, and OCR values. Many cancer cells produce ATP through the glycolytic pathway. On the other hand, it has been recently reported that cancer cells whose glycolytic pathway is suppressed survive by shifting their energy metabolism to OXPHOS by enhancing mitochondrial function, and the dependency of metabolic pathways differs depending on cell lines. |
|||
<Evaluation by Lactate production and ATP levels>We confirmed the changes in ATP and Lactate production when ATP synthesis by OXPHOS was inhibited by Oligomycin stimulation and by 2-Deoxy-D-glucose (2-DG) in the glycolytic pathway. The results showed that HeLa cells depend on Glycolysis and HepG2 cells depend on OXPHOS to synthesize ATP. |
<Evaluation by Lactate production and ATP levels>
Products in Use |
||
<Evaluation by OCR value> |
Using the same number of cells, we measured the OCR value when cellular oxygen consumption was promoted by stimulating the cells with FCCP, a mitochondrial uncoupling agent. The results showed that HepG2 cells had higher OCR values than HeLa cells, suggesting a greater dependence on OXPHOS, correlating with the results obtained from ATP level and Lactate production. Products in Use |
||
Lipid Droplet Accumulation Disrupts Cell Homeostasis and Contributes to Disease [Aug. 13, 2024]
Open / Close the Article
|
Lipid droplets (LDs) play an important role in several diseases, including cancer, neurodegeneration and cellular senescence. In cancer, LD accumulation is often observed and can support rapid cell proliferation by providing essential lipids for membrane synthesis and energy production. Neurodegenerative diseases such as Alzheimer's and Parkinson's show aberrant lipid metabolism, with excessive LDs contributing to neuroinflammation and neuronal dysfunction. In cellular senescence, lipid droplets sequester polyunsaturated fatty acids to prevent ferroptosis, a type of cell death, thereby impacting aging and age-related diseases. |
|||
| Acetyl-CoA carboxylase obstructs CD8+ T cell lipid utilization in the tumor microenvironment Click here for the original article: Elizabeth Hunt, et. al., Cell Metabolism, 2024. |
Cell cycle arrest induces lipid droplet formation and confers ferroptosis resistance Click here for the original article: Hyemin Lee, et. al., Nature Communications, 2024. |
Senescent glia link mitochondrial dysfunction and lipid accumulation Click here for the original article: China Byrns, et. al., Nature, 2024. |
|
|
Point of Interest - The solid TME activates acetyl-coenzyme A carboxylase (ACC) in T cells, causing lipid droplet accumulation in tumor-infiltrating T cells, which inhibits FAO. - Restricting ACC activity rewires T cell metabolism to maintain energy during TME stress. |
Point of Interest - DGAT inhibition reshuffles PUFAs to phospholipids, which resensitizes arrested cells to ferroptosis. - Some slow-cycling antimitotic drug–resistant cancer cells, such as 5-fluorouracil–resistant cells, have accumulation of lipid droplets and combined treatment with ferroptosis inducers and DGAT inhibitors effectively suppresses their growth. |
Point of Interest - AP1+ senescent glia cause lipid accumulation in non-senescent glia and increase senescence markers. - Targeting AP1 in senescent glia extends lifespan, but increases oxidative damage in the brain and neuronal mitochondrial function remains poor. |
|
| Related Techniques | |||
| Lipid Droplet Detection Kit | Lipid Droplet Assay Kit - Blue / Deep Red | ||
| Lipid Droplet Staining | Lipi-Blue/ Green/ Red/ Deep Red | ||
| Ferrous ion (Fe2+) detection | FerroOrange(intracellular), Mito-FerroGreen(mitochondrial) | ||
| Lipid peroxidation detection | Liperfluo(intracellular), MitoPeDPP(mitochondrial) | ||
| Glutathione Quantification | GSSG/GSH Quantification Kit | ||
| Cystine Uptake detection | Cystine Uptake Assay Kit | ||
| Cell Cycle Measurement | Cell Cycle Assay Solution Blue / Deep Red | ||
| Cellular senescence detection | SPiDER-βGal for live-cell imaging or flow cytometry / microplate reader / tissue samples | ||
| Mttochondrial membrane potential detection | JC-1 MitoMP Detection Kit, MT-1 MitoMP Detection Kit | ||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | ||
| Related Applications | |||
Hepatotoxicity test of drug-induced lipidosis using high-content imagingPropranolol (a sympathetic β-receptor blocker) was added to a human hepatocellular carcinoma cell line (HepG2 cells), and changes in lipid droplets were observed under a fluorescence microscope. The accumulation of lipid droplets was analyzed by measuring the number, area, and fluorescence intensity of lipid droplets from the acquired microscopic images. |
|||
<Lipid droplet imaging data>
Nucleus (blue: Hoechst33342 ): Ex 385 nm, Em 460 nm |
HepG2 cells were treated with propranolol 0, 10, or 30 μmol/l, lipid droplets were stained with Lipi-Green and nuclei with Hoechst 33342 and observed using a fluorescence microscope (Ti2-E inverted microscope). The results showed that lipid droplets increased in a propranolol concentration-dependent manner.
Related Products |
||
<Analysis of lipid droplet accumulation relative to drug treatment concentration>
For details of staining and analysis methods, please refer to "APPLICATION NOTE: Hepatotoxicity test of drug-induced lipidosis using high-content imaging" by Nikon Corporation. |
From the fluorescence images obtained, the accumulation of lipid droplets per cell was analyzed by measuring cell number from nuclei and area, number, and fluorescence intensity from lipid droplets. The results showed that the number and area of lipid droplets increased in a propranolol concentration-dependent manner, with lipid droplets forming significantly under concentration conditions of 20 μmol/l or higher. The DS-Qi2 camera, which can capture a wide range of cellular areas in a single shot, was used for imaging, and the EDF module of NIS-Elements software, which can acquire focused images of all lipid droplets, was used for analysis, enabling quantitative analysis with highly reliable statistical data. The EDF module of the NIS-Elements software allows for the acquisition of focused images of all lipid droplets. |
||
Lactate: Multifunctional Metabolite in Cellular Function [Jan. 30, 2024]
Open / Close the Article
|
Lactate, best known as a metabolite in the body, plays several key roles. It is primarily produced during anaerobic metabolism, such as the Warburg effect in cancer cells, as a byproduct of the conversion of glucose to energy when oxygen levels are low. Beyond its role in energy metabolism, lactate acts as a signaling molecule, influencing various cellular processes such as gene expression and inflammation regulation. In addition, recent research suggests that lactate may serve as an alternative energy source for the brain and other tissues, challenging the traditional view of lactate as a waste product. |
|||
| Metabolic regulation of homologous recombination repair by MRE11 lactylation Click here for the original article: Yuping Chen, et. al., Cell, 2024. |
Lactate modulates iron metabolism by binding soluble adenylyl cyclase Click here for the original article: Wei Liu, et. al., Cell Metabolism, 2023. |
Lactate activates the mitochondrial electron transport chain independently of its metabolism Click here for the original article: Xin Cai, et. al., Molecular Cell, 2023. |
|
|
Point of Interest - The lactylation of MRE11 facilitates DNA end breakage and homologous recombination repair. - Elevated lactate levels in cancer cells lead to the lactylation of MRE11, contributing to chemoresistance. - Targeting the lactylation of MRE11 can increase the sensitivity of cancer cells to chemotherapy. |
Point of Interest - Lactate directly binds to soluble adenylyl cyclase, enhancing its enzymatic activity. - Lactate modulates iron homeostasis by inducing the expression of hepatic hepcidin. |
Point of Interest - It activates the electron transport chain (ETC) independently of its own metabolism. - Activation of the ETC by lactate results in increased pyruvate oxidation and enhanced lactate utilization. - The mitochondrial ETC can sense the availability of lactate and recognize it as a potential nutrient. |
|
| Related Techniques | |||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | ||
| Mitochondrial function/glycolysis detection | Glycolysis/JC-1 MitoMP Assay Kit NEW | ||
| Oxygen consumption rate assay | Extracellular OCR Plate Assay Kit | ||
| Glycolysis-related metabolites assay | Glucose and Lactate Assay Kit | ||
| TCA cycle-related metabolites assay | Glutamine, Glutamate, and α-KG Assay Kit | ||
| NAD(H) and NADP(H) redox couples assay | NAD/NADH and NADP/NADPH Assay Kit | ||
| Antibody/Protein labeling with fast and high recovery | Fluorescein, Biotin, and Peroxidase Labeling Kit - NH2 | ||
| Related Applications | |||
Comparison of metabolic pathway in two types of cancer cellsThe dependence of OXPHOS and Glycolysis in two types of cancer cells, HeLa and HepG2, were compared based on Lactate production, ATP levels, and OCR values. Many cancer cells produce ATP through the glycolytic pathway. On the other hand, it has been recently reported that cancer cells whose glycolytic pathway is suppressed survive by shifting their energy metabolism to OXPHOS by enhancing mitochondrial function, and the dependency of metabolic pathways differs depending on cell lines. |
|||
<Evaluation by Lactate production and ATP levels>We confirmed the changes in ATP and Lactate production when ATP synthesis by OXPHOS was inhibited by Oligomycin stimulation and by 2-Deoxy-D-glucose (2-DG) in the glycolytic pathway. The results showed that HeLa cells depend on Glycolysis and HepG2 cells depend on OXPHOS to synthesize ATP. |
<Evaluation by Lactate production and ATP levels>
Products in Use |
||
<Evaluation by OCR value> |
Using the same number of cells, we measured the OCR value when cellular oxygen consumption was promoted by stimulating the cells with FCCP, a mitochondrial uncoupling agent. The results showed that HepG2 cells had higher OCR values than HeLa cells, suggesting a greater dependence on OXPHOS, correlating with the results obtained from ATP level and Lactate production. Products in Use |
||
Metabolic Alterations Controlling Cancer Cell Fate [May. 7, 2024]
Open / Close the Article
|
Metabolic alterations play a key role in determining the fate of cancer cells. Dysregulated metabolism, often characterized by increased glucose uptake and altered nutrient utilization, provides cancer cells with the necessary energy and building blocks necessary for rapid proliferation. In addition, metabolic reprogramming can confer resistance to apoptosis and promote tumor progression. Targeting these metabolic vulnerabilities holds promise for the development of therapeutic strategies that selectively eradicate cancer cells while sparing normal tissues. Understanding the intricate interplay between metabolism and cancer cell fate is essential for advancing personalized cancer therapies. |
|||
| Acetyl-CoA carboxylase 1 controls a lipid droplet–peroxisome axis and is a vulnerability of endocrine-resistant ER+ breast cancer Click here for the original article: Marina Bacci, et. al., Science Translational Medicine, 2024. |
Numb/Parkin-directed mitochondrial fitness governs cancer cell fate via metabolic regulation of histone lactylation Click here for the original article: Yuman He, et. al., Cell Reports, 2023. |
Phospholipids with two polyunsaturated fatty acyl tails promote ferroptosis Click here for the original article: Baiyu Qiu, et. al., Cell, 2024. |
|
|
Point of Interest - Lipid droplets together with peroxisomes, which are enriched and active in the LTED cells, conferred metabolic adaptability to the resistant tumors. - This reprogramming was controlled by acetyl-CoA-carboxylase-1 (ACC1), whose targeting ACC1 selectively impaired LTED survival. - Targeting ACC1 reduced tumor growth of resistant patient-derived xenografts. |
Point of Interest - Disruption of mitochondrial quality control triggers a metabolic-epigenetic switch. - Histone lactylation induces transcriptional upregulation of neuroendocrine genes. - Dysfunction in the Numb/Parkin axis propels a neuroendocrine cell fate in cancer cells. |
Point of Interest -PC-PUFA2s interacted with the mitochondrial electron transport chain, generating ROS to initiate lipid peroxidation. -Mitochondria-targeted antioxidants protected cells from PC-PUFA2-induced mtROS, lipid peroxidation, and cell death. |
|
| Related Techniques | |||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | ||
| Mitochondrial function/glycolysis detection | Glycolysis/JC-1 MitoMP Assay Kit | ||
| Oxygen consumption rate assay | Extracellular OCR Plate Assay Kit | ||
| Mitochondria Detection | Mitophagy Detection Kit, MitoBright ROS Deep Red | ||
| Ferroptosis: Mitochondrial / internal iron detection | Mito-FerroGreen, FerroOrange | ||
| Lipid Droplet detection | Lipid Droplet Assay Kit - Blue / Deep Red | ||
| Lysosomal function | Acidic pH Detection Kit-Green/Red and Green/Deep Red | ||
| Cellular senescence detection | SPiDER-βGal for live-cell imaging or flow cytometry / microplate reader / tissue samples | ||
| NAD(H) and NADP(H) redox couples assay | NAD/NADH and NADP/NADPH Assay Kit | ||
| Related Applications | |||
Comparison of metabolic pathway in two types of cancer cellsThe dependence of OXPHOS and Glycolysis in two types of cancer cells, HeLa and HepG2, were compared based on Lactate production, ATP levels, and OCR values. Many cancer cells produce ATP through the glycolytic pathway. On the other hand, it has been recently reported that cancer cells whose glycolytic pathway is suppressed survive by shifting their energy metabolism to OXPHOS by enhancing mitochondrial function, and the dependency of metabolic pathways differs depending on cell lines. |
|||
<Evaluation by Lactate production and ATP levels>We confirmed the changes in ATP and Lactate production when ATP synthesis by OXPHOS was inhibited by Oligomycin stimulation and by 2-Deoxy-D-glucose (2-DG) in the glycolytic pathway. The results showed that HeLa cells depend on Glycolysis and HepG2 cells depend on OXPHOS to synthesize ATP. |
<Evaluation by Lactate production and ATP levels>
Products in Use |
||
<Evaluation by OCR value> |
Using the same number of cells, we measured the OCR value when cellular oxygen consumption was promoted by stimulating the cells with FCCP, a mitochondrial uncoupling agent. The results showed that HepG2 cells had higher OCR values than HeLa cells, suggesting a greater dependence on OXPHOS, correlating with the results obtained from ATP level and Lactate production. Products in Use |
||
Neuronal somatic aerobic glycolysis in maintaining antioxidant capacity [Dec. 5, 2023]
Open / Close the Article
|
Scientists have discovered that in both basal and activated states, neuronal somata have higher levels of aerobic glycolysis and lower levels of OXPHOS than terminals. Their findings update the conventional view that neurons use OXPHOS uniformly under basal conditions and highlight the important role of somatic aerobic glycolysis in maintaining antioxidant capacity. |
|||
|
Aerobic glycolysis is the predominant means of glucose metabolism in neuronal somata, which protects against oxidative damage |
|||
|
Point of Interest |
|||
| Related Techniques | |||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | ||
| Mitochondrial function/glycolysis detection | Glycolysis/JC-1 MitoMP Assay Kit | ||
| Oxygen consumption rate assay | Extracellular OCR Plate Assay Kit | ||
| Glycolysis-related metabolites assay | Glucose and Lactate Assay Kit | ||
| TCA cycle-related metabolites assay | Glutamine, Glutamate, and α-KG Assay Kit | ||
| NAD(H) and NADP(H) redox couples assay | NAD/NADH and NADP/NADPH Assay Kit | ||
| Total ROS detection | Highly sensitive DCFH-DA or Photo-oxidation Resistant DCFH-DA | ||
| Related Applications | |||
Comparison of metabolic pathway in two types of cancer cellsThe dependence of OXPHOS and Glycolysis in two types of cancer cells, HeLa and HepG2, were compared based on Lactate production, ATP levels, and OCR values. Many cancer cells produce ATP through the glycolytic pathway. On the other hand, it has been recently reported that cancer cells whose glycolytic pathway is suppressed survive by shifting their energy metabolism to OXPHOS by enhancing mitochondrial function, and the dependency of metabolic pathways differs depending on cell lines. |
|||
<Evaluation by Lactate production and ATP levels>
|
We confirmed the changes in ATP and Lactate production when ATP synthesis by OXPHOS was inhibited by Oligomycin stimulation and by 2-Deoxy-D-glucose (2-DG) in the glycolytic pathway. The results showed that HeLa cells depend on Glycolysis and HepG2 cells depend on OXPHOS to synthesize ATP. <Evaluation by Lactate production and ATP levels> Products in Use |
||
<Evaluation by OCR value>
|
Using the same number of cells, we measured the OCR value when cellular oxygen consumption was promoted by stimulating the cells with FCCP, a mitochondrial uncoupling agent. The results showed that HepG2 cells had higher OCR values than HeLa cells, suggesting a greater dependence on OXPHOS, correlating with the results obtained from ATP level and Lactate production. Products in Use |
||
Aerobic Glycolysis Impact on Tumor's Sensitivity to T-Cell Killing [Nov. 7, 2023]
Open / Close the Article
|
Scientists have found that deficiency of two key glycolytic enzymes, Glut1 (glucose transporter 1) and Gpi1 (glucose-6-phosphate isomerase 1), enhanced tumor cell killing by cytotoxic T cells. Genetic and pharmacologic inactivation of Glut1 sensitizes tumors to anti-tumor immunity and synergizes with anti-PD-1 therapy through the TNF-α pathway. |
|||
|
Tumor aerobic glycolysis confers immune evasion through modulating sensitivity to T cell-mediated bystander killing via TNF-α |
|||
|
Point of Interest |
|||
| Related Techniques | |||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | ||
| Nutrient uptake Assay | Glucose (Blue / Green / Red), Amino Acid, Cystine, and Fatty Acid Uptake Assay Kit | ||
| Glycolysis-related metabolites assay | Glucose and Lactate Assay Kit | ||
| TCA cycle-related metabolites assay | Glutamine, Glutamate, and α-KG Assay Kit | ||
| NAD(H) and NADP(H) redox couples assay | NAD/NADH and NADP/NADPH Assay Kit | ||
| Mitochondrial function/glycolysis detection | Glycolysis/JC-1 MitoMP Assay Kit | ||
| Oxygen consumption rate assay | Extracellular OCR Plate Assay Kit | ||
| Related Applications | |||
Metabolic shift to glycolysis in senescenct cells |
|||
<Evaluation by Lactate production and ATP levels>
|
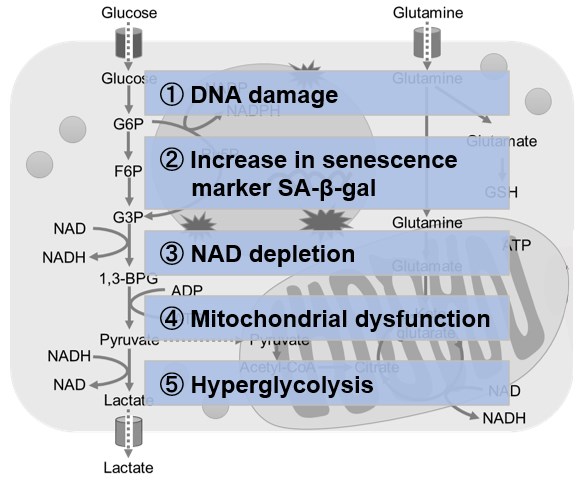
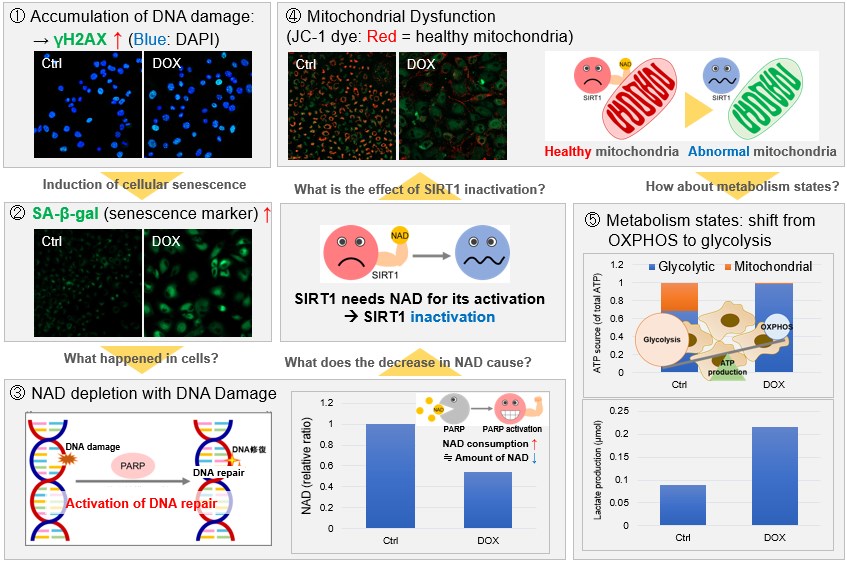
NAD(+) levels decline during the aging process, causing defects in nuclear and mitochondrial functions and resulting in many age-associated pathologies*. Here, we try to redemonstrate this phenomenon in the doxorubicin (DOX)-induced cellular senescence model with a comprehensive analysis of our products. *S. Imai, et al., Trends Cell Biol, 2014, 24, 464-471
|
||
|
|
|||
Lysosomal Cystine Governs Ferroptosis [Oct. 24, 2023]
Open / Close the Article
|
Scientists have found that lysosomal cystine deprivation, unlike general amino acid starvation, triggers ATF4 induction at the transcriptional level, but cytosolic cysteine deprivation does not. Blocking lysosomal cystine efflux diminishes ATF4 induction and enhances sensitivity to ferroptosis. Therefore, intracellular nutrient reprogramming could potentially induce selective ferroptosis in cancer cells without causing systemic starvation. |
|||
|
Lysosomal cystine governs ferroptosis sensitivity in cancer via cysteine stress response |
|||
|
Point of Interest |
|||
| Related Techniques | |||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | ||
| Lysosomal function assay |
Lysosomal Acidic pH Detection Kit-Green/Red |
||
| Nutrient uptake Assay | Glucose (Blue / Green / Red), Amino Acid, Cystine, and Fatty Acid Uptake Assay Kit | ||
| Glycolysis-related metabolites assay | Glucose and Lactate Assay Kit | ||
| TCA cycle-related metabolites assay | Glutamine, Glutamate, and α-KG Assay Kit | ||
| Mitochondrial function/glycolysis detection | Glycolysis/JC-1 MitoMP Assay Kit | ||
| Oxygen consumption rate assay | Extracellular OCR Plate Assay Kit | ||
| Related Applications | |||
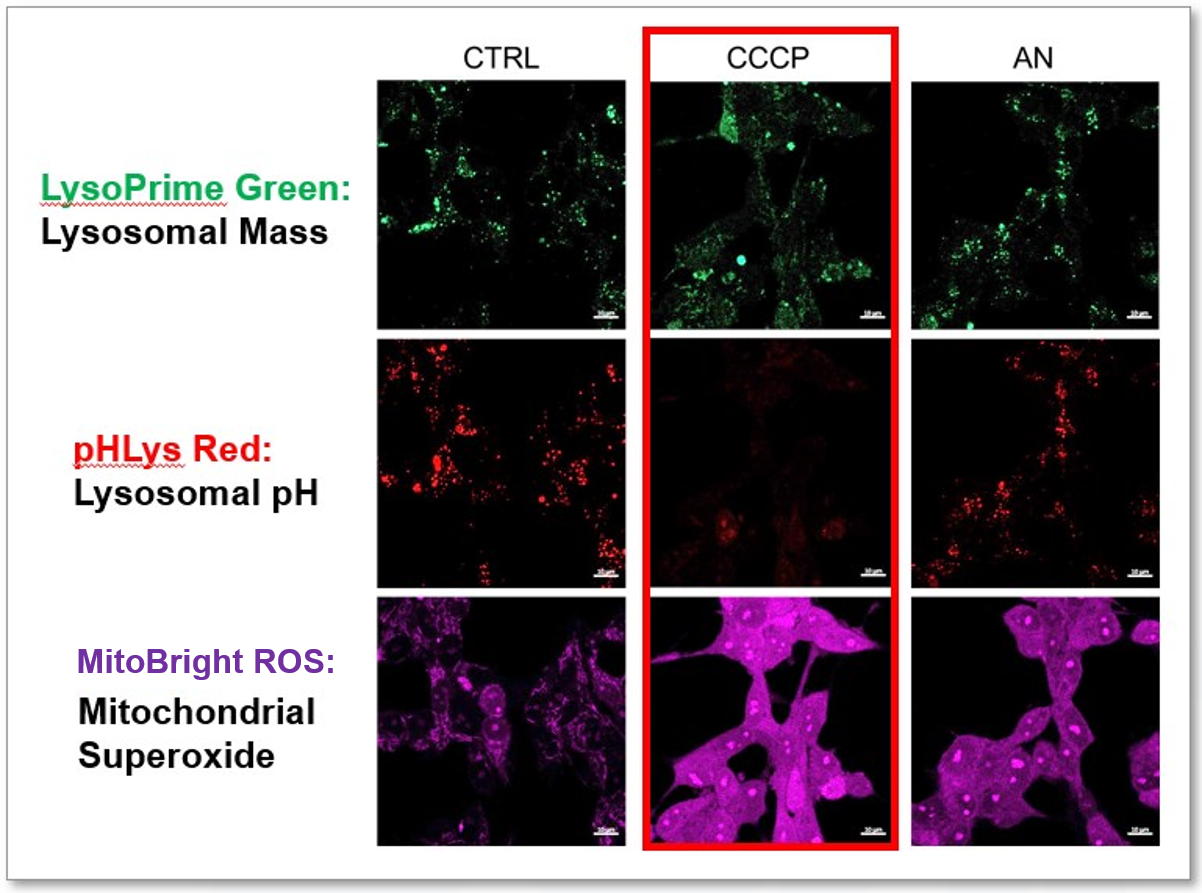
Lysosomal Function and Mitochondrial ROS
|
CCCP and Antimycin are recognized inducers of mitochondrial ROS, linked to the loss of mitochondrial membrane potential. Recent studies have shown that CCCP induces not only mitochondrial ROS but also lysosomal dysfunction. To observe mitochondrial ROS, HeLa cells were labeled with MitoBright ROS Deep Red for Mitochondrial Superoxide Detection, and the lysosomal mass and pH were independently detected with LysoPrime Green and pHLys Red. Co-staining with MitoBright ROS and Lysosomal dyes revealed that CCCP, unlike Antimycin, triggers concurrent lysosomal neutralization and mitochondrial ROS induction. Reference: Benjamin S Padman, et. al., Autophagy (2013) Products in Use |
||
Lysosomal Function and Iron Homeostasis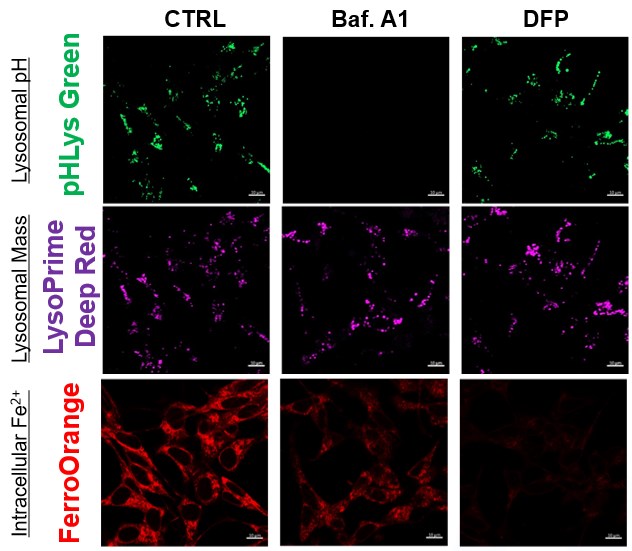 |
Recent reports suggest that lysosomal neutralization can result in iron depletion, consequently leading to the disruption of cell viability. To verify this, HeLa cells were labeled with FerroOrange for Fe2+ detection, and the lysosomal mass and pH were separately detected with LysoPrime DeepRed and pHLys Green (a product currently under development). Co-staining with FerroOrange and Lysosomal dyes demonstrated that Bafilomycin A1 (Baf. A1), an inhibitor of lysosomal acidification, causes iron depletion consistent with the findings reported in the article. Interestingly, the iron chelator, Deferiprone (DFP), did not impact lysosomal pH, suggesting that lysosomal function plays a key role in managing iron homeostasis. Reference: Ross A Weber, et. al., Mol Cell (2020) Products in Use *pHLys Green is a component of "Lysosomal Acidic pH Detection Kit-Green/Deep Red". |
||
Targeting the TCA Cycle: A Novel Approach to Neuroinflammation [Oct. 31, 2023]
Open / Close the Article
|
Scientists have found that neuroinflammatory lesions in the mouse spinal cord cause widespread and persistent axonal ATP deficiency that precedes mitochondrial oxidation and calcium overload. Notably, viral overexpression of individual TCA enzymes can ameliorate axonal energy deficits in neuroinflammatory lesions. This suggests that TCA cycle dysfunction in the common neuroinflammatory disease multiple sclerosis may be amenable to therapy. |
|||
|
Targeting the TCA cycle can ameliorate widespread axonal energy deficiency in neuroinflammatory lesions |
|||
|
Point of Interest |
|||
| Related Techniques | |||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | ||
| Fatty acid uptake assay | Fatty Acid Uptake Assay Kit | ||
| Lipid droplet detection | Lipi-Blue / Green / Red / Deep Red | ||
| Glycolysis-related metabolites assay | Glucose and Lactate Assay Kit | ||
| TCA cycle-related metabolites assay | Glutamine, Glutamate, and α-KG Assay Kit | ||
| Mitochondrial function/glycolysis detection | Glycolysis/JC-1 MitoMP Assay Kit | ||
| Oxygen consumption rate assay | Extracellular OCR Plate Assay Kit | ||
| Related Applications | |||
Comparison of metabolic pathway in two types of cancer cellsThe dependence of OXPHOS and Glycolysis in two types of cancer cells, HeLa and HepG2, were compared based on Lactate production, ATP levels, and OCR values. Many cancer cells produce ATP through the glycolytic pathway. On the other hand, it has been recently reported that cancer cells whose glycolytic pathway is suppressed survive by shifting their energy metabolism to OXPHOS by enhancing mitochondrial function, and the dependency of metabolic pathways differs depending on cell lines. |
|||
<Evaluation by Lactate production and ATP levels>
|
We confirmed the changes in ATP and Lactate production when ATP synthesis by OXPHOS was inhibited by Oligomycin stimulation and by 2-Deoxy-D-glucose (2-DG) in the glycolytic pathway. The results showed that HeLa cells depend on Glycolysis and HepG2 cells depend on OXPHOS to synthesize ATP. <Evaluation by Lactate production and ATP levels> Products in Use |
||
<Evaluation by OCR value>
|
Using the same number of cells, we measured the OCR value when cellular oxygen consumption was promoted by stimulating the cells with FCCP, a mitochondrial uncoupling agent. The results showed that HepG2 cells had higher OCR values than HeLa cells, suggesting a greater dependence on OXPHOS, correlating with the results obtained from ATP level and Lactate production. Products in Use |
||
Metabolic Maturation Shapes Cardiomyocyte Epigenetics in Heart [Oct. 17, 2023]
Open / Close the Article
|
Scientists have discovered that disablement of fatty acid oxidation in cardiomyocytes improves resistance to hypoxia and stimulates cardiomyocyte proliferation, allowing heart regeneration after ischaemia–reperfusion injury. This process involves epigenetic inhibition of cardiomyocyte maturation via activation of the alpha-ketoglutarate-dependent lysine demethylase KDM5. |
|||
|
Inhibition of fatty acid oxidation enables heart regeneration in adult mice |
|||
|
Point of Interest |
|||
| Related Techniques | |||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | ||
| Fatty acid uptake assay | Fatty Acid Uptake Assay Kit | ||
| Lipid droplet detection | Lipi-Blue / Green / Red / Deep Red | ||
| Glycolysis-related metabolites assay | Glucose and Lactate Assay Kit | ||
| TCA cycle-related metabolites assay | Glutamine, Glutamate, and α-KG Assay Kit | ||
| Mitochondrial function/glycolysis detection | Glycolysis/JC-1 MitoMP Assay Kit | ||
| Oxygen consumption rate assay | Extracellular OCR Plate Assay Kit | ||
| Related Applications | |||
Fatty acid starvation induced by uptake inhibitor evoke reprogramming of cellular metabolism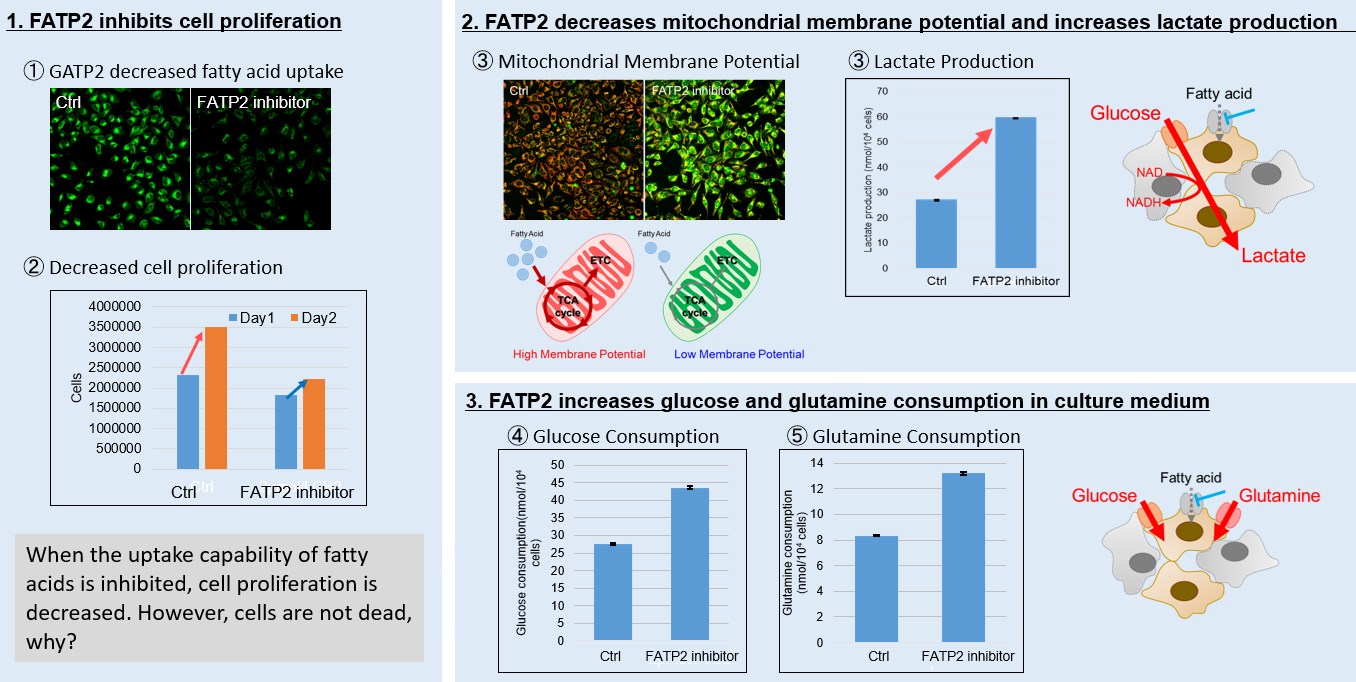
Mitochondrial fatty acid β-oxidation and oxidative phosphorylation (OXPHOS) are crucial biochemical processes that metabolize fats and sugars to produce ATP, the cell's primary energy source. In this section, we underscored the significance of fatty acid starvation and energy pathways, with an emphasis on the fatty acid uptake inhibitor, FATP2. Here are the key findings from our experiments conducted on HeLa cells: ・Inhibition of fatty acid uptake results in reduced cell proliferation, though it does not lead to cell death. This was determined through the use of a Cell Counting Kit-8 and Fatty Acid Uptake Kit (Image 1). ・Fatty acid starvation shifts cellular metabolism from OXPHOS to glycolysis, as indicated by the Glycolysis/JC-1 MitoMP Assay Kit. (Image 2) ・When fatty acid uptake is inhibited, a compensatory increase in glucose and glutamine uptake occurs to preserve cell viability, as observed using the Glucose Assay Kit and Glutamine Assay Kit. (Image 3) Products in Use for Cell Proliferation/Cytotoxicity Assay for Glycolysis Assay and Mitochondrial Membrane Potential Detection for Glucose and Glutamine Consumption Assay |
|||
Lipid-mitochondria Axis in Metabolism [Aug 8, 2023]
Open / Close the Article
|
In recent years, the discovery of several novel lipid-mitochondria axis in metabolism has attracted much attention. A link between acylcarnitine accumulation and lipid intolerance in skeletal muscle mitochondria is tightly linked to poor health. In other breakthroughs, decreased expression in fatty acid transporters and β-oxidation enzymes causes fatty acid buildup in lipid droplets. Research also uncovers that knocking out a vital enzyme in mitochondrial fatty acid oxidation astrocytes leads to cognitive impairment. |
||
|
Pyruvate-supported flux through medium-chain ketothiolase promotes mitochondrial lipid tolerance in cardiac and skeletal muscles |
Arf1 coordinates fatty acid metabolism and mitochondrial homeostasis |
Fatty acid oxidation organizes mitochondrial supercomplexes to sustain astrocytic ROS and cognition |
|
Point of Interest |
Point of Interest |
Point of Interest |
| Related Techniques | ||
| Lipid droplets detection | Lipi-Blue / Green / Red / Deep Red | |
| Fatty acid uptake assay | Fatty Acid Uptake Assay Kit | |
| Mitochondrial function/glycolysis detection | Glycolysis/JC-1 MitoMP Assay Kit | |
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit | |
| Oxygen consumption rate assay | Extracellular OCR Plate Assay Kit | |
| Mitochondrial superoxide detection by deep red staining, co-staining with other markers | MitoBright ROS Deep Red - Mitochondrial Superoxide Detection | |
| NAD(H) and NADP(H) redox couples assay | NAD/NADH and NADP/NADPH Assay Kit | |
| Related Applications | ||
Fatty acid starvation induced by uptake inhibitor evoke reprogramming of cellular metabolism
|
||
|
Mitochondrial fatty acid β-oxidation and oxidative phosphorylation (OXPHOS) are crucial biochemical processes that metabolize fats and sugars to produce ATP, the cell's primary energy source. In this section, we underscored the significance of fatty acid starvation and energy pathways, with an emphasis on the fatty acid uptake inhibitor, FATP2. Here are the key findings from our experiments conducted on HeLa cells: ・Inhibition of fatty acid uptake results in reduced cell proliferation, though it does not lead to cell death. This was determined through the use of a Cell Counting Kit-8 and Fatty Acid Uptake Kit (Image 1). ・Fatty acid starvation shifts cellular metabolism from OXPHOS to glycolysis, as indicated by the Glycolysis/JC-1 MitoMP Assay Kit. (Image 2) ・When fatty acid uptake is inhibited, a compensatory increase in glucose and glutamine uptake occurs to preserve cell viability, as observed using the Glucose Assay Kit and Glutamine Assay Kit. (Image 3) |
Products in Use for Cell Proliferation/Cytotoxicity Assay for Glycolysis Assay and Mitochondrial Membrane Potential Detection for Glucose and Glutamine Consumption Assay |
|
Glycolysis-related Mitophagy Mitigates the Parkinson's Disease Phenotypes [Jun. 6, 2023]
Open / Close the Article
| Scientists have unveiled that the loss of ubiquitin carboxyl-terminal hydrolase L1 (UCHL1; also called PARK5) regarded as an important Parkinson's Disease (PD)-associated gene mitigates the PD-related phenotypes via induction of mitophagy. Remarkably, UCHL1-controlled mitophagy is triggered by the suppression of PKM resulting in the inhibition of glycolysis. This occurs independently of the PINK1-Parkin pathway. The study suggests that comprehensive regulation of glucose metabolism and mitochondrial homeostasis may aid in suppressing the pathogenesis of PD. Learn more about how the authors detect mitophagy with Mitophagy Detection Kit (refer to Figure 2B). | |
|
Loss of UCHL1 rescues the defects related to Parkinson’s disease by suppressing glycolysis Point of Interest |
|
| Related Techniques | |
| Glycolytic/Mitochondrial activity detection | Glycolysis/JC-1 MitoMP Assay Kit NEW |
| Mitophagy detection | Mitophagy Detection Kit |
| Lysosomal function assay | Lysosomal pH and mass detection Kit |
| Autophagy detection | DAPGreen / DAPRed (Autophagosome detection), DALGreen (Autolysosome detection) |
| Oxygen consumption rate assay | Extracellular OCR Plate Assay Kit HOT |
| Related Applications | |
Fatty acid starvation induced by uptake inhibitor evoke reprogramming of cellular metabolism
Mitochondrial fatty acid β-oxidation and oxidative phosphorylation (OXPHOS) are crucial biochemical processes that metabolize fats and sugars to produce ATP, the cell's primary energy source. In this section, we underscored the significance of fatty acid starvation and energy pathways, with an emphasis on the fatty acid uptake inhibitor, FATP2. Here are the key findings from our experiments conducted on HeLa cells: ・Inhibition of fatty acid uptake results in reduced cell proliferation, though it does not lead to cell death. This was determined through the use of a Cell Counting Kit-8 and Fatty Acid Uptake Kit (Image 1). ・Fatty acid starvation shifts cellular metabolism from OXPHOS to glycolysis, as indicated by the Glycolysis/JC-1 MitoMP Assay Kit. (Image 2) ・When fatty acid uptake is inhibited, a compensatory increase in glucose and glutamine uptake occurs to preserve cell viability, as observed using the Glucose Assay Kit and Glutamine Assay Kit. (Image 3) Products in Use for Cell Proliferation/Cytotoxicity Assay for Glycolysis Assay and Mitochondrial Membrane Potential Detection
|
|
Neuronal Coordination of Nutrient-Associated Genes and Brain Development [Apr. 4, 2023]
Open / Close the Article
| This article focusing on how mammalian neurons coordinate the expression of a nutrient-associated gene with the regulation of neuronal activity to ensure proper brain development. We believe that these findings can offer valuable insights into human brain metabolism. | |
|
Large neutral amino acid levels tune perinatal neuronal excitability and survival Point of Interest |
|
| Related Techniques | |
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit HOT |
| Oxygen Consumption Rate (OCR) Assay | Extracellular OCR Plate Assay Kit HOT |
| Glycolysis-related metabolites assay | Glucose and Lactate Assay Kit |
| TCA cycle-related metabolites assay | Glutamine, Glutamate, and α-KG Assay Kit |
| NAD(H) and NADP(H) redox couples assay | NAD/NADH and NADP/NADPH Assay Kit |
| ATP Assay | ATP Assay Kit and ADP/ATP Assay Kit |
| Nutrient uptake Assay | Glucose (Blue / Green / Red), Amino Acid, Cystine, and Fatty Acid Uptake Assay Kit HOT |
| Lipid droplets detection | Lipi-Blue / Green / Red / Deep Red |
| Related Applications | |
The metabolic shift to glycolysis under the treatment of fatty acid transporter inhibitors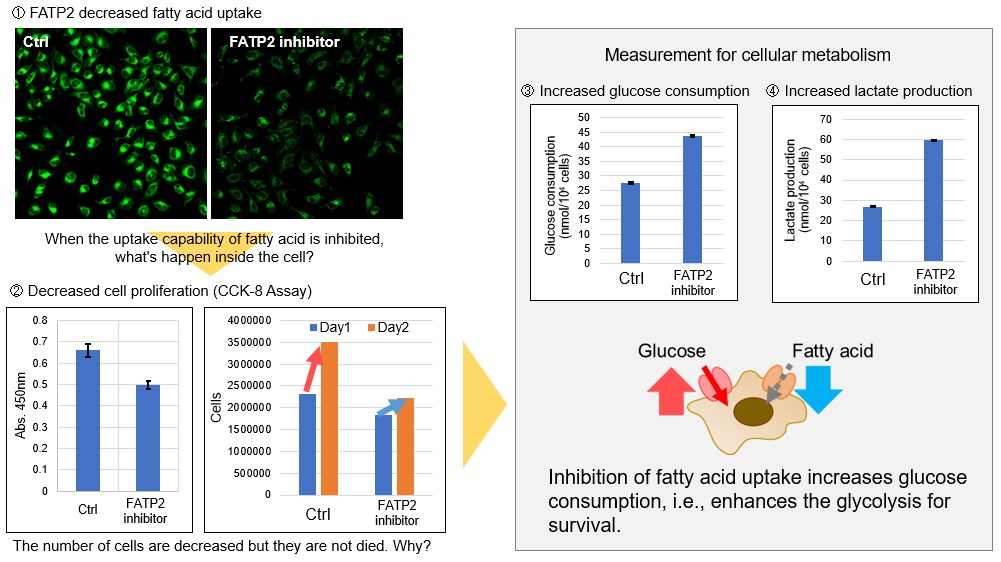
Products in Use for Cell Proliferation/Cytotoxicity Assay for Glycolysis Assay
|
|
Lactylation-dependent metabolic adaptation [Mar. 14, 2023]
Open / Close the Article
| This article focusing on what type of posttranslational modification, lactilation, plays an important role in regulating cellular metabolism and may contribute to hepatocellular carcinoma progression. These findings may suggest the possibility that the functional effect of Lactilation not only in cancer cells but also the phenomena where metabolic shifts to glycolysis occur such as cellular senescence. | |||||
|
Lactylome analysis suggests lactylation-dependent mechanisms of metabolic adaptation in hepatocellular carcinoma Point of Interest |
|||||
| Related Techniques | |||||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit HOT | ||||
| Oxygen Consumption Rate (OCR) Assay | Extracellular OCR Plate Assay Kit HOT | ||||
| Glycolysis-related metabolites assay | Glucose and Lactate Assay Kit | ||||
| TCA cycle-related metabolites assay | Glutamine, Glutamate, and α-KG Assay Kit | ||||
| NAD(H) and NADP(H) redox couples assay | NAD/NADH and NADP/NADPH Assay Kit | ||||
| ATP Assay | ATP Assay Kit | ||||
| Nutrient uptake Assay | Glucose (Blue / Green / Red), Amino Acid, Cystine, and Fatty Acid Uptake Assay Kit HOT | ||||
| Lipid droplets detection | Lipi-Blue / Green / Red / Deep Red | ||||
| Related Applications | |||||
Metabolic shift to glycolysis in senescenct cells
Products in Use |
|||||
Unexpected cellular metabolism impacts on diseases [Jan. 31, 2023]
Open / Close the Article
| Regulation of cellular metabolism is critical for maintaining the proper balance of energy and biomolecules within the cell. Hormones, enzymes, and other regulatory molecules control the rate of metabolic reactions. Imbalances in cellular metabolism can lead to diseases such as diabetes, cancer, and metabolic disorders. Interestingly, several findings are beginning to be reported that intracellular metabolism contributes to cellular homeostasis in unexpected ways. Today, we introduce you to three highlighted articles focusing on cellular metabolism related to TCA cycle in nucleus, Metabolic transformation, Lipid- controlled mitochondrial transfer. | ||
|---|---|---|
| TCA cycle in nucleus | Warburg-like metabolic transformation in Alzheimer’s disease | Dietary lipids and mitochondria transfer |
| Operation of a TCA cycle subnetwork in the mammalian nucleus (Eleni Kafkia, et al., Science Avvances, 8, eabq5206, 2022) |
Warburg-like metabolic transformation underlies neuronal degeneration in sporadic Alzheimer’s disease (Larissa Traxler, et al., Cell Metabolism, 34, 1248-1263, 2022) |
Dietary lipids inhibit mitochondria transfer to macrophages to divert adipocyte-derived mitochondria into the blood (Nicholas Borcherding, et al., Cell Metabolism, 34, 1499-1513, 2022) |
|
|
|
| Related Technique in This Topic | ||
| Glycolysis/Oxidative phosphorylation Assay | Glycolysis/OXPHOS Assay Kit HOT | |
| Oxygen Consumption Rate (OCR) Assay | Extracellular OCR Plate Assay Kit HOT | |
| Glycolysis-related metabolites assay | Glucose and Lactate Assay Kit | |
| TCA cycle-related metabolites assay | Glutamine, Glutamate, and α-KG Assay Kit | |
| NAD(H) and NADP(H) redox couples assay | NAD/NADH and NADP/NADPH Assay Kit | |
| ATP Assay | ATP Assay Kit | |
| Nutrient uptake Assay | Glucose (Blue / Green / Red), Amino Acid, Cystine, and Fatty Acid Uptake Assay Kit HOT | |
| Lipid droplets detection | Lipi-Blue / Green / Red / Deep Red | |