Fatty Acid Uptake Assay Kit
Fatty Acid Uptake Assay
-
Product codeUP07 Fatty Acid Uptake Assay Kit
| Unit size | Price | Item Code |
|---|---|---|
| 100 tests | $416.00 | UP07-10 |
Approximate number of uses
1 set for one 96-well plate
| 100 tests | ・Fatty Acid Uptake Probe ・Quenching Buffer ・Washing Buffer (10X) |
×1 11 ml×1 11 ml×1 |
|---|
Description
This kit uses Fatty Acid Uptake Probe as a fatty acid analog, which can be uptaken into cells via fatty acid transporters on the cell membrane surface. Cellular fatty acids uptake ability can be evaluated by the fluorescence intensity with fluorescence microscopies, flow cytometers, plate readers or other fluorescence instruments. In addition, a reagent that eliminates the fluorescence of any extracellular fatty acid analog is included, so it is possible to easily measure the actual fatty acid uptake capability even if washing the cells is difficult.
Manual
Technical info
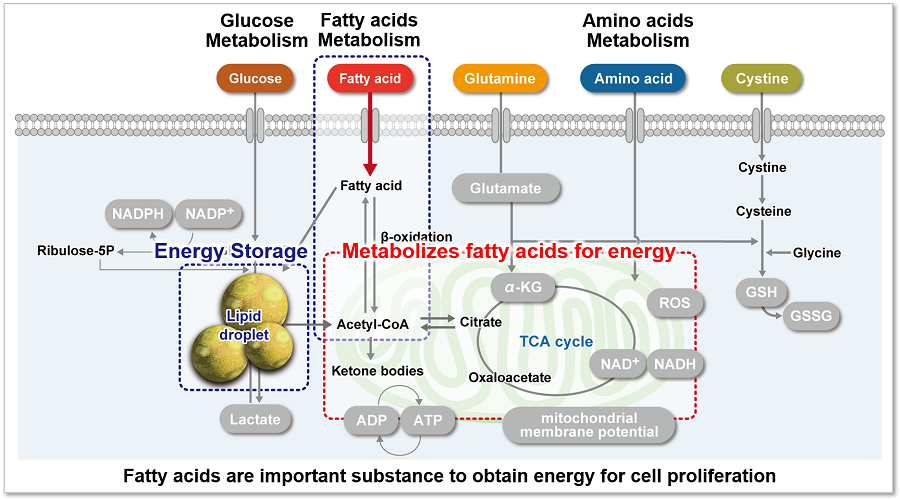
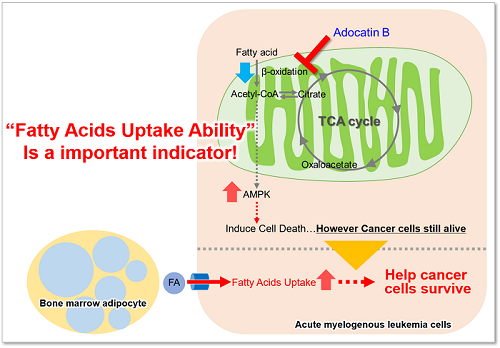
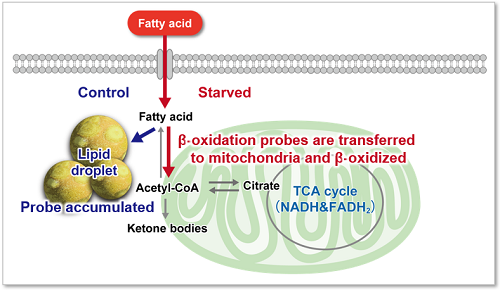
Fatty acids are important for the body to obtain energy. Fatty acid uptake ability is not only related to diseases such as obesity and diabetes but is also one of the metabolic indicators in cancer cells (upper figure). Cancer cells, which are proliferating rapidly, require large amounts of lipids, and therefore, intracellular fatty acid synthesis and uptake of fatty acids from the medium are actively taking place (lower figure). Therefore, many cancer therapies have been developed to target the fatty acid metabolic pathway in cancer cells.
This kit solves it all! Technique Issues in Fatty Acid Uptake Experiments
This kit addresses all of the following comments and problems from fatty acid uptake researchers.
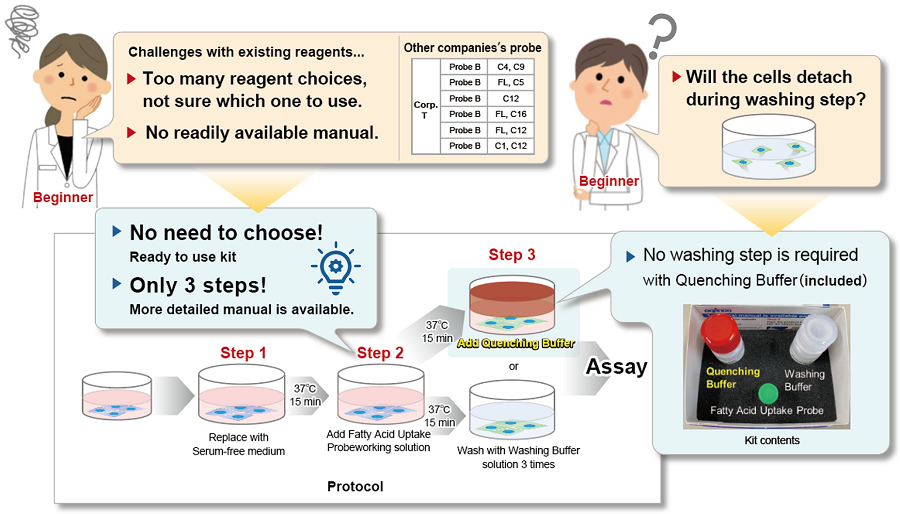
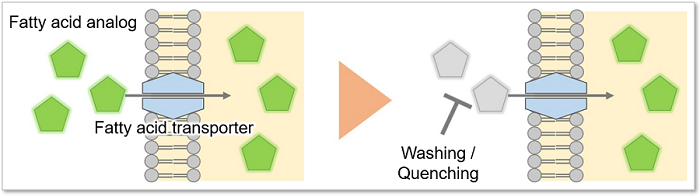
This kit contains a fatty acid analog (Fatty Acid Uptake Probe) which can be uptaken by cells via fatty acid transporters, and fatty acid uptake ability can be detected by a fluorescence method (Principle). The Quenching Buffer enables detection without cell-washing steps (Protocol).
Principle
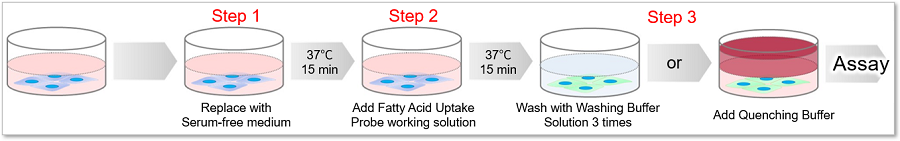
Protocol
How to choose between Washing Buffer and Quenching Buffer?
Please refer to the table below and select either Washing Buffer or Quenching Buffer according to the cell type and instruments.
| Washing Buffer (10×) | Quenching Buffer | ||||
|---|---|---|---|---|---|
| Adherent cell | Floating cell | Adherent cell | Floating cell | ||
| Cell-washing Procedure | Necessary | Unnecessary | |||
| Plate Reader | Bottom Reading (Clear Bottom) | Applicable | Applicable | Conditionally*1 | |
| Top Reading | Applicable | Inapplicable | |||
| Fluorescene Microscope | Applicable | Applicable | |||
| Confocal Microscope | Applicable | Conditionally*2 | |||
| Flow Cytometer | Applicable | Inapplicable | |||
*1 The cell number needs to be sufficient to cover the bottom of the well(Approx. 3x105 cells/well), after the cells settled on the bottom of the well, it is possible to assay.
*2When using a confocal microscope, transmitted light mode with 488 nm is not possible. When performing transmitted light observation, please dilute the Quenching Buffer 10-fold with Washing Buffer or using a 640 nm laser.
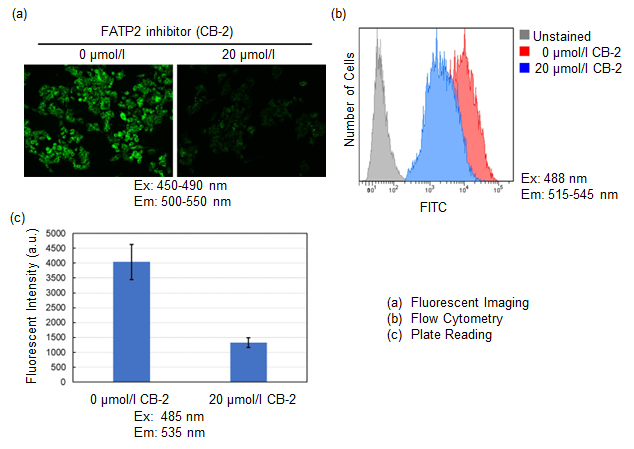
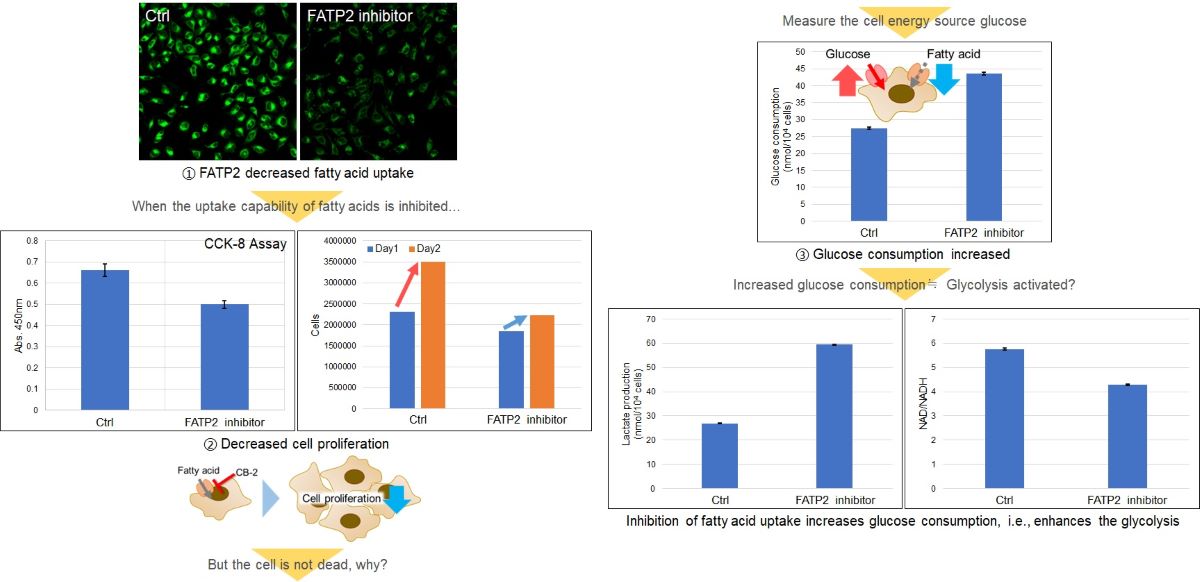
Inhibition of fatty acid uptake by fatty acid uptake inhibitor:CB-2
Using this kit, we were able to observe and quantify the inhibition of fatty acid uptake in HepG2 cells by a fatty acid transporter inhibitor (FATP2 inhibitor: CB-2). Please refer to the instruction manual for details of the experimental procedure.
Application Data: Evaluation of Fatty Acid Metabolism
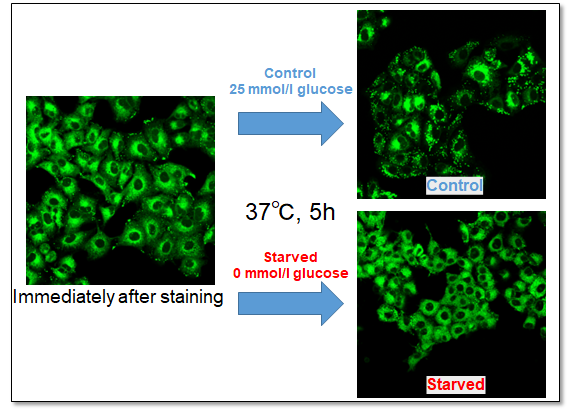
A549 cells were stained with this kit and observed under a fluorescence microscope, followed by further 5h incubation in medium containing 0 mmol/l glucose (Starved) or 25 mmol/l glucose (Control).
Immediately after staining, the probe was localized throughout the cytoplasm. While it was localized mainly in lipid droples when the cells were incubated in medium containing 25 mmol/l glucose (Control). On the other hand, glucose starvation treatment (Starved) showed the probe localization in mitochondria and endoplasmic reticulum, suggesting enhanced β-oxidation. The product was confirmed to be able to detect changes in fatty acid metabolism after uptake.
<Observation Condition>
Filter set: GFP (Ex = 450 - 490 nm, Em = 500 - 550 nm)
Application Data: Alteration of intracellular metabolism of HeLa cells by treatment with fatty acid transporter inhibitors
Fatty acids are important for membrane synthesis and are essential for cell proliferation. HeLa cells were treated with a fatty acid transporter inhibitor, and then the cell proliferation and intracellular metabolism (glucose consumption, Lactate release, and NAD/NADH ratio) were tested.
The results showed a decrease in cell proliferation, an increase in glucose consumption and Lactate release, and a decrease in the intracellular NAD+/NADH ratio, indicating a shift in the metabolic pathway to glycolysis.
<Related Products>
Cell Proliferation: Cell Counting Kit-8 (Code: CK04)
Glucose: Glucose Assay Kit-WST (Code: G264)
Lactate: Lactate Assay Kit-WST (Code: L256)
NAD/NADH Ratio: NAD/NADH Assay Kit-WST (Code: N509)
Q & A
-
Q
How many cell lines has been tested with this kit?
-
A
We tested the following cell lines with this kit.
Cell Line Derived from A549 Human alveolar basal epithelial cells HepG2 Human liver epithelialcells HeLa Human cervical cancer cells Jurkat Human T lymphocyte cells MOLT-4 Human acute lymphoblastic leukemia cells 3T3-L1 Preadipocyte 3T3-L1 Adipocyte
-
Q
Is it possible to fix cells after staining with the Fatty Acid Uptake Probe?
-
A
We have tested fixing cells after staining with 4% PFA.
〈Protocol〉
-Adherent cell-
1. Prepared the cell in a dish or microtube.
2. Removed the medium, and washed the cells twice with serum-free medium.
3. Added serum-free medium, incubated at 37℃, 5% CO2 for 15 min.
4. Removed the medium, Added Fatty Acid Uptake Probe working solution, then incubated at 37℃, 5% CO2 for 15 min.
5. Removed the medium, and washed the cells once with Washing Buffer solution.
6. Added 4% PFA/PBS to the cells, incubated 5 min at room temperature.
7. Washed the cells 3 times with PBS, and then tested the samples with a fluorescence microscope and a plate reader.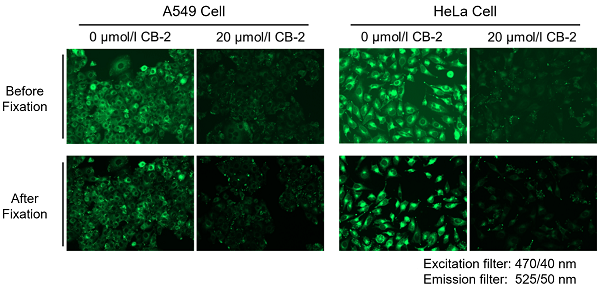
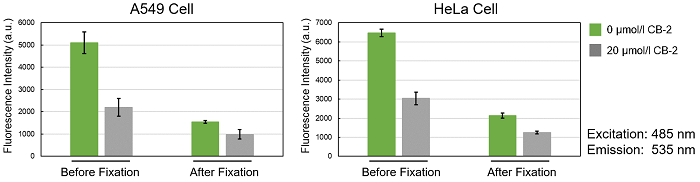
*Please be aware that cell fixation may decrease the fluorescence intensity.
-Floating cell-
1. Prepared the cells in a microtube.
2. Centrifuged at 300×g for 5 min and then removed the supernatant.
3. Added serum-free medium, after suspending the cells by pipetting, centrifuged at 300×g for 5 min, and then removed the supernatant. Repeated this step once.
4. Added serum-free medium, after suspending the cells by pipetting, incubated at 37℃, 5% CO2 for 15 min.
5. Centrifuged at 300×g for 5 min, and then removed the supernatant.
6. Added Fatty Acid Uptake Probe working solution, after suspending the cells by pipetting, incubated at 37℃, 5% CO2 for 15 min.
7. Centrifuged at 300×g for 5 min, and then removed the supernatant.
8. Washed the cells once with the Washing Buffer solution.
9. Added 4% PFA/PBS, after suspending the cells by pipetting, incubated 5 min at room temperature.
10. Centrifuged at 300×g for 5 mins, and then removed the supernatant.
11. Added PBS, after suspending the cells by pipetting, centrifuged at 300×g for 5 min, and then removed the supernatant. Repeated this step twice.
12. Tested the samples with a fluorescence microscope and a flow cytometer.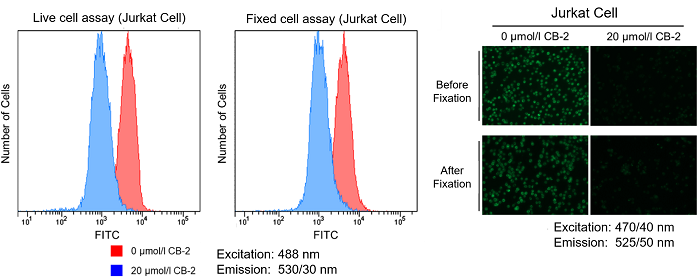
-
Q
Which kind of plate should I use when measuring with a plate reader?
-
A
Please use a black microplate for fluorescence assay.
Adherent cell Floating cell Opaque bottom
PlateClear bottom
PlateOpaque bottom
PlateClear bottom
Plate
Washing Buffer (10×)
ProcedureTop Reading Applicable Applicable Bottom Reading Inapplicable Applicable Inapplicable Applicable Quenching Buffer
ProcedureTop Reading Inapplicable Inapplicable Bottom Reading Inapplicable Applicable Inapplicable Conditionally* *The cell number needs to be sufficient to cover the bottom of the well, after the cells settled on the bottom of the well, it is possible to assay.〈Floating Cells Sample using Quenching Buffer Procudure〉
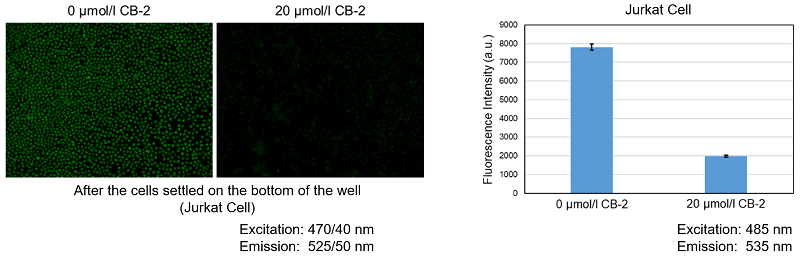
We have also tested with the following microplates.
Company Product Name Cat No. ibidi µPlate 96 well ibiTreat black S 15 ib89626 Thermo Fisher 96 Well Black/Clear Bottom Plate, TC Surface, Pack of 10 165305
-
Q
Can I store the Fatty Acid Uptake Probe working solution for a long period of time?
-
A
The Fatty Acid Uptake Probe working solution can't be stored for a long period of time.
-
Q
What should I do if the background is high?
-
A
One of the possible reasons is that there was some of the Fatty Acid Uptake Probe still remaining in the well, please wash the cells one more time with the Washing Buffer solution or consider using the Quenching Buffer procedure.
-
Q
How many samples can be measured with this kit?
-
A
Please refer to the following table.
Adherent cell Floating cell Container (Volume) 6-well (1.5 ml/well) 24-well (0.3 ml/well) 96-well (0.1 ml/well) 35-mm dish (1.5 ml/well) 1.5-ml microtube (0.5 ml/tube) Samples can be tested 7 sample 34 sample 100 sample 7 sample 20 sample
-
Q
When I used the transmitted light mode of confocal microscope during the Quenching Buffer protocol, there was nothing on the screen. What should I do?
-
A
When using a confocal microscope, transmitted light mode with 488 nm is not possible. When performing transmitted light observation, please dilute the Quenching Buffer 10-fold with Washing Buffer or using a 640 nm laser.
〈Image of the transmitted light mode of confocal microscope〉
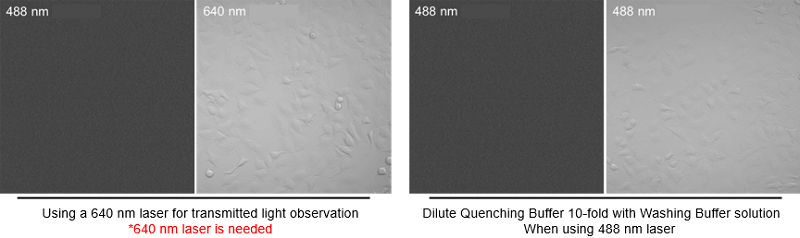
-
Q
Can I use this kit to quantify fatty acid?
-
A
This kit can't quantify fatty acid.
-
Q
Can I use this kit to quantify the uptaken Fatty Acid Uptake Probe inside the cells?
-
A
You can't quantify the uptaken Fatty Acid Uptake Probe. This kit is for fatty acid uptake ability evaluation.
-
Q
Can I co-stain with other fluorescent probe at the same time?
-
A
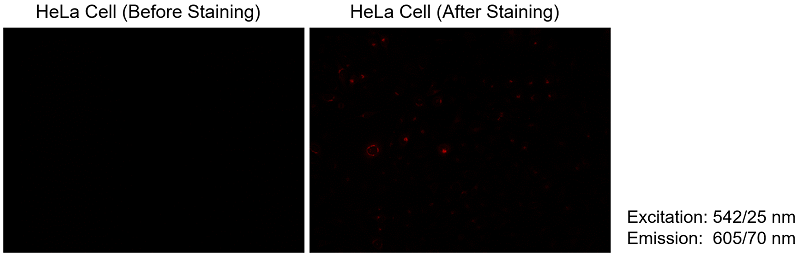
The Fatty Acid Uptake Probe shows a slight leakage to red fluorescence. Therefore, please use dyes other than green and red fluorescence for co-staining.
We have tested using mitochondrial fluorescence probe MitoBright LT Deep Red (MT12) for co-staining.
〈Red fluorescence leakage〉
〈Protocol of MitoBright LT Deep Red co-staining〉
1. Prepared the cells in a dish or microtube.
2. Removed the medium, and then washed the cells twice with serum-free medium.
3. Added serum-free medium, incubated at37℃, 5%CO2 for 15 min.
4. Removed the supernatant, added Fatty Acid Uptake Probe working solution, and then incubated at 37℃, 5%CO2 for 15 min.
5. Removed the supernatant, added 0.1 µmol/l MitoBright LT working solution, and then incubated at 37℃, 5%CO2 for 30 min.
6. Removed the supernatant, and washed the cells twice with HBSS.
7. Added HBSS, and then observed with a fluorescence microscope.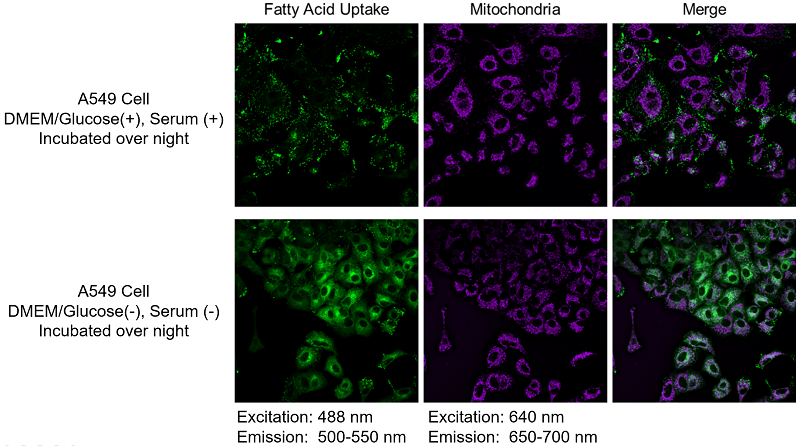
-
Q
Is there any tips or precautions when using a fluorescence microscope?
-
A
During fluorescence imaging, continuous exposure to excitation light may cause photobleaching of the Fatty Acid Uptake Probe. Please refrain from continuous irradiation.
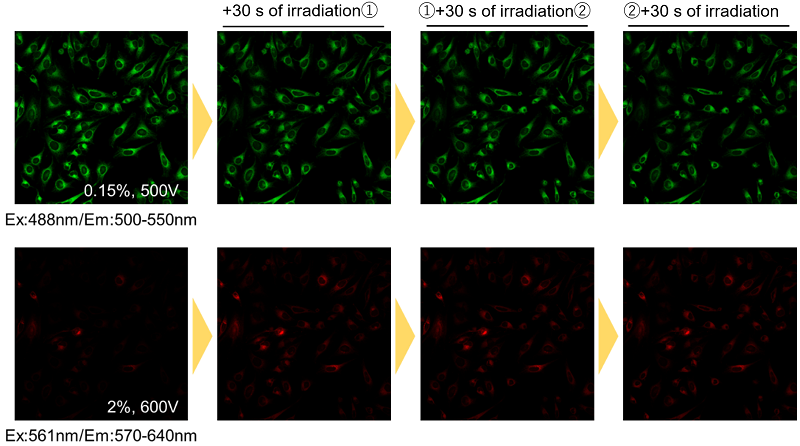
Handling and storage condition
| 0-5°C |