|
Scientists have discovered that in both basal and activated states, neuronal somata have higher levels of aerobic glycolysis and lower levels of OXPHOS than terminals. Their findings update the conventional view that neurons use OXPHOS uniformly under basal conditions and highlight the important role of somatic aerobic glycolysis in maintaining antioxidant capacity.
|
-
Aerobic glycolysis is the predominant means of glucose metabolism in neuronal somata, which protects against oxidative damage
Click here for the original article: Yao Wei, et. al., Nature Neuroscience, 2023.
Point of Interest
- Neuronal somata engage more in aerobic glycolysis and less in OXPHOS compared to axon terminals, both at rest and when activated.
- The enzyme pyruvate kinase 2 (PKM2) is primarily found in neuronal somata, not terminals.
- Deleting Pkm2 in mice shifts somatic metabolism from aerobic glycolysis to OXPHOS.
- This shift causes oxidative damage and loss of dopaminergic neurons.
|
| Related Techniques |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Related Applications |
Comparison of metabolic pathway in two types of cancer cells
The dependence of OXPHOS and Glycolysis in two types of cancer cells, HeLa and HepG2, were compared based on Lactate production, ATP levels, and OCR values.
Many cancer cells produce ATP through the glycolytic pathway. On the other hand, it has been recently reported that cancer cells whose glycolytic pathway is suppressed survive by shifting their energy metabolism to OXPHOS by enhancing mitochondrial function, and the dependency of metabolic pathways differs depending on cell lines.
|
<Evaluation by Lactate production and ATP levels>
-
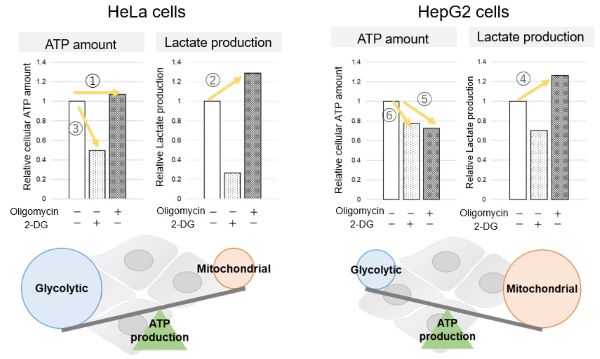
We confirmed the changes in ATP and Lactate production when ATP synthesis by OXPHOS was inhibited by Oligomycin stimulation and by 2-Deoxy-D-glucose (2-DG) in the glycolytic pathway. The results showed that HeLa cells depend on Glycolysis and HepG2 cells depend on OXPHOS to synthesize ATP.
-
When OXPHOS was inhibited in HeLa cells, ATP levels remained unchanged (①), and lactate production increased (②). This suggests that even when OXPHOS is inhibited, glycolysis can be further activated. Conversely, when glycolysis is inhibited, ATP levels decrease significantly (③), indicating that energy production depends on glycolysis. On the other hand, when OXPHOS was inhibited in HepG2 cells, lactate production increased (④), indicating that the cells attempt to compensate for energy production by enhancing glycolysis, but ATP levels still decrease (⑤). This means that even with increased glycolysis, ATP production is not sufficiently compensated. Furthermore, ATP levels decrease more when glycolysis is inhibited (⑥), suggesting that energy production in HepG2 cells depends more on OXPHOS than glycolysis.
Products in Use
- Glycolysis/OXPHOS Assay Kit
-
<Evaluation by OCR value>
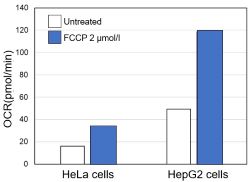 
-
Using the same number of cells, we measured the OCR value when cellular oxygen consumption was promoted by stimulating the cells with FCCP, a mitochondrial uncoupling agent. The results showed that HepG2 cells had higher OCR values than HeLa cells, suggesting a greater dependence on OXPHOS, correlating with the results obtained from ATP level and Lactate production.
Products in Use
- Extracellular OCR Plate Assay Kit
|


















