|
Metabolic alterations play a key role in determining the fate of cancer cells. Dysregulated metabolism, often characterized by increased glucose uptake and altered nutrient utilization, provides cancer cells with the necessary energy and building blocks necessary for rapid proliferation. In addition, metabolic reprogramming can confer resistance to apoptosis and promote tumor progression. Targeting these metabolic vulnerabilities holds promise for the development of therapeutic strategies that selectively eradicate cancer cells while sparing normal tissues. Understanding the intricate interplay between metabolism and cancer cell fate is essential for advancing personalized cancer therapies.
|
-
Acetyl-CoA carboxylase 1 controls a lipid droplet–peroxisome axis and is a vulnerability of endocrine-resistant ER+ breast cancer
Click here for the original article: Marina Bacci, et. al., Science Translational Medicine, 2024.
Point of Interest
- Analysis of long-term estrogen-deprived (LTED) ER+ breast cancer cells, a model of aromatase inhibitor resistance, revealed enhanced intracellular lipid storage.
- Lipid droplets together with peroxisomes, which are enriched and active in the LTED cells, conferred metabolic adaptability to the resistant tumors.
- This reprogramming was controlled by acetyl-CoA-carboxylase-1 (ACC1), whose targeting ACC1 selectively impaired LTED survival.
- Targeting ACC1 reduced tumor growth of resistant patient-derived xenografts.
-
Numb/Parkin-directed mitochondrial fitness governs cancer cell fate via metabolic regulation of histone lactylation
Click here for the original article: Yuman He, et. al., Cell Reports, 2023.
Point of Interest
- Numb, a cell fate determinant, binds to Parkin and facilitates Parkin-mediated mitophagy.
- Disruption of mitochondrial quality control triggers a metabolic-epigenetic switch.
- Histone lactylation induces transcriptional upregulation of neuroendocrine genes.
- Dysfunction in the Numb/Parkin axis propels a neuroendocrine cell fate in cancer cells.
-
Phospholipids with two polyunsaturated fatty acyl tails promote ferroptosis
Click here for the original article: Baiyu Qiu, et. al., Cell, 2024.
Point of Interest
-An accumulation of diacyl-PUFA phosphatidylcholines (PC-PUFA2s) after fatty acid or phospholipid treatments correlates with cancer cell sensitivity to ferroptosis.
-PC-PUFA2s interacted with the mitochondrial electron transport chain, generating ROS to initiate lipid peroxidation.
-Mitochondria-targeted antioxidants protected cells from PC-PUFA2-induced mtROS, lipid peroxidation, and cell death.
|
| Related Techniques |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
| Related Applications |
Comparison of metabolic pathway in two types of cancer cells
The dependence of OXPHOS and Glycolysis in two types of cancer cells, HeLa and HepG2, were compared based on Lactate production, ATP levels, and OCR values.
Many cancer cells produce ATP through the glycolytic pathway. On the other hand, it has been recently reported that cancer cells whose glycolytic pathway is suppressed survive by shifting their energy metabolism to OXPHOS by enhancing mitochondrial function, and the dependency of metabolic pathways differs depending on cell lines.
|
<Evaluation by Lactate production and ATP levels>
-
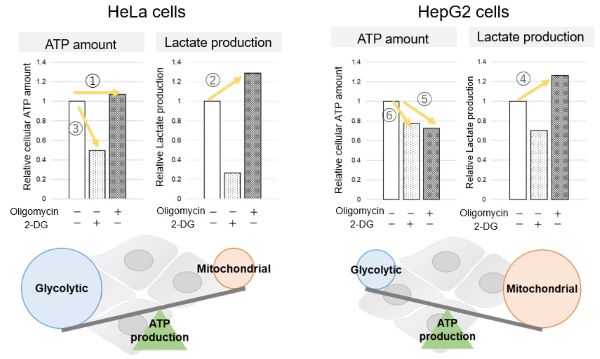
We confirmed the changes in ATP and Lactate production when ATP synthesis by OXPHOS was inhibited by Oligomycin stimulation and by 2-Deoxy-D-glucose (2-DG) in the glycolytic pathway. The results showed that HeLa cells depend on Glycolysis and HepG2 cells depend on OXPHOS to synthesize ATP.
-
When OXPHOS was inhibited in HeLa cells, ATP levels remained unchanged (①), and lactate production increased (②). This suggests that even when OXPHOS is inhibited, glycolysis can be further activated. Conversely, when glycolysis is inhibited, ATP levels decrease significantly (③), indicating that energy production depends on glycolysis. On the other hand, when OXPHOS was inhibited in HepG2 cells, lactate production increased (④), indicating that the cells attempt to compensate for energy production by enhancing glycolysis, but ATP levels still decrease (⑤). This means that even with increased glycolysis, ATP production is not sufficiently compensated. Furthermore, ATP levels decrease more when glycolysis is inhibited (⑥), suggesting that energy production in HepG2 cells depends more on OXPHOS than glycolysis.
Products in Use
- Glycolysis/OXPHOS Assay Kit
-
<Evaluation by OCR value>
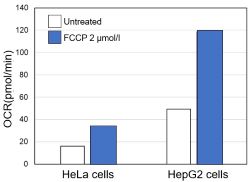 
-
Using the same number of cells, we measured the OCR value when cellular oxygen consumption was promoted by stimulating the cells with FCCP, a mitochondrial uncoupling agent. The results showed that HepG2 cells had higher OCR values than HeLa cells, suggesting a greater dependence on OXPHOS, correlating with the results obtained from ATP level and Lactate production.
Products in Use
- Extracellular OCR Plate Assay Kit
|


















