ABD-F
Derivatization Reagent for HPLC
-
Product codeA016 ABD-F
-
CAS No.91366-65-3
-
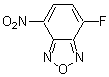
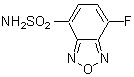
Chemical name4-Fluoro-7-sulfamoylbenzofurazan
-
MWC6H4FN3O3S=217.18
| Unit size | Price | Item Code |
|---|---|---|
| 50 mg | $302.00 | A016-10 |
| 100 mg | $481.00 | A016-12 |
Description
Derivatization Reaction
Product Description
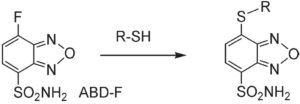
ABD-F has a benzofurazan moiety that produces a highly fluorescent compound through the reaction with a sulfhydryl group. The excitation and emission of the devivatized compound are 389 nm and 513 nm, respectively. The reaction rate of ABD-F is 30 times faster than that of SBD-F. ABD-F reactions with thiol compounds are completed within 5 minutes in aqueous conditions at 50ºC, pH 8. However, ABD-F does not react with alanine, proline, or cystine under these conditions. Its maximum fluorescence intensity can be observed at pH 2. In reversephase HPLC analysis, pre-labeled ABD-thiol compounds can be detected separately. The detection limits (S/N=3) are 0.6 pmol per injection for cysteine, 0.4 pmol per injection for glutathione, 1.9 pmol per injection for N-acetylcysteine, and 0.5 pmol per injection for cysteamine.
Technical info
ABD Labeling Protocol
1. To prepare sample solution, mix or dissolve a sample with 100 mM borate buffer, pH 8.0 containing 2 mM EDTA.
2. Mix 500 μl of the sample solution and 500 μl of 1 mM ABD-F/100 mM borate buffer in a reaction vial.
3. Heat the vial at 50ºC for 5 minutes and cool it on an ice bath.
4. Add 300 μl of 100 mM HCl aqueous solution to the reaction mixture.
5. Use this mixture for HPLC analysis to determine ABD-labeled compounds; excitation: 389 nm, emission: 513 nm.
References
1) T. Toyo'oka and K. Imai, "New Fluorogenic Reagent Having Halogenobenzofurazan Structure for Thiols: 4-(Aminosulfonyl)-7-fluoro-2,1,3-benzoxadiazole", Anal. Chem., 1984, 56, 2461.
2) T. Toyo'oka and K. Imai, "Isolation and Characterization of Cysteine-Containing Regions of Proteins Using 4-(Aminosulfonyl)-7-fluoro-2,1,3-benzoxadiazole and High-Performance Liquid Chromatography", Anal. Chem., 1985, 57, 1931.
3) T. Toyo'oka, H. Miyano and K. Imai, "Amino Acid Composition Analysis of Minute Amounts of Cysteine-containing Proteins Using 4-(Aminosulfonyl)-7-fluoro-2,1,3-benzoxadiazole and 4-fluoro-7-nitro-2,1,3-benzoxadiazole in Combination with HPLC", Biomed. Chromatogr., 1986, 1, 15.
4) T. Toyo'oka, S. Uchiyama and Y. Saito, "Simultaneous Determination of Thiols and Disulfides by High-performance Liquid Chromatography with Fluorescence Detection", Anal. Chim. Acta, 1988, 205, 29.
5) Y. Luo, Y. Zhou and B. S. Cooperman, "Antichymotrypasin Interaction with Chymotrypsin", J. Biol., Chem., 1999, 274(25), 17733.
Handling and storage condition
| Appearance: | White to slightly yellow crystalline powder |
|---|---|
| Purity (HPLC): | ≧ 99.0 % |
| Solubility in acetonitrile: | To pass test (clear, slightly yellow) |
| Molar absorptivity: | ≧ 4,500 (around 315 nm) |
| m.p.: | 140 - 149oC |
| IR spectrum: | Authentic |
| -20°C, Protect from light, Protect from moisture |