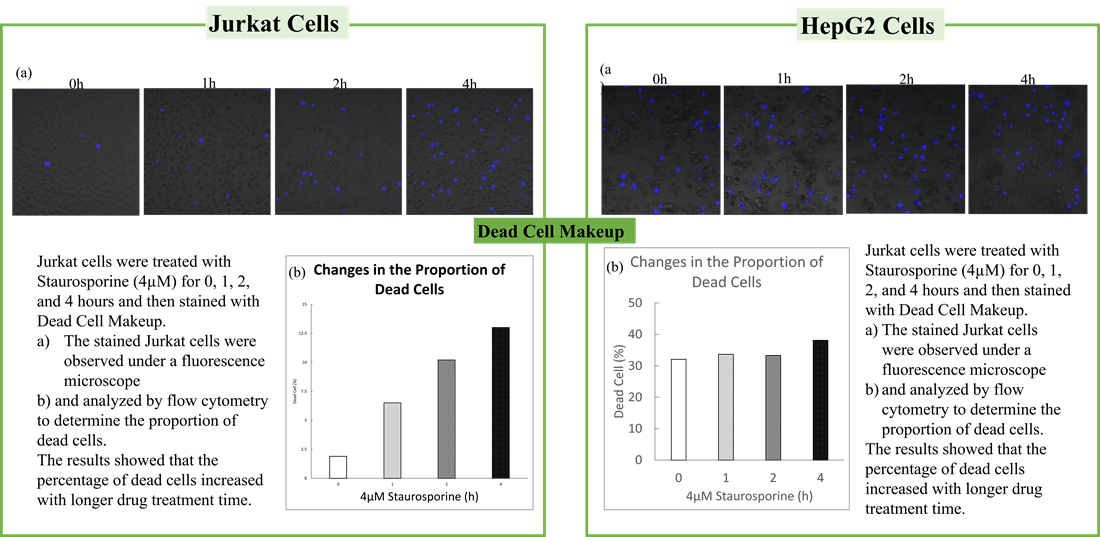
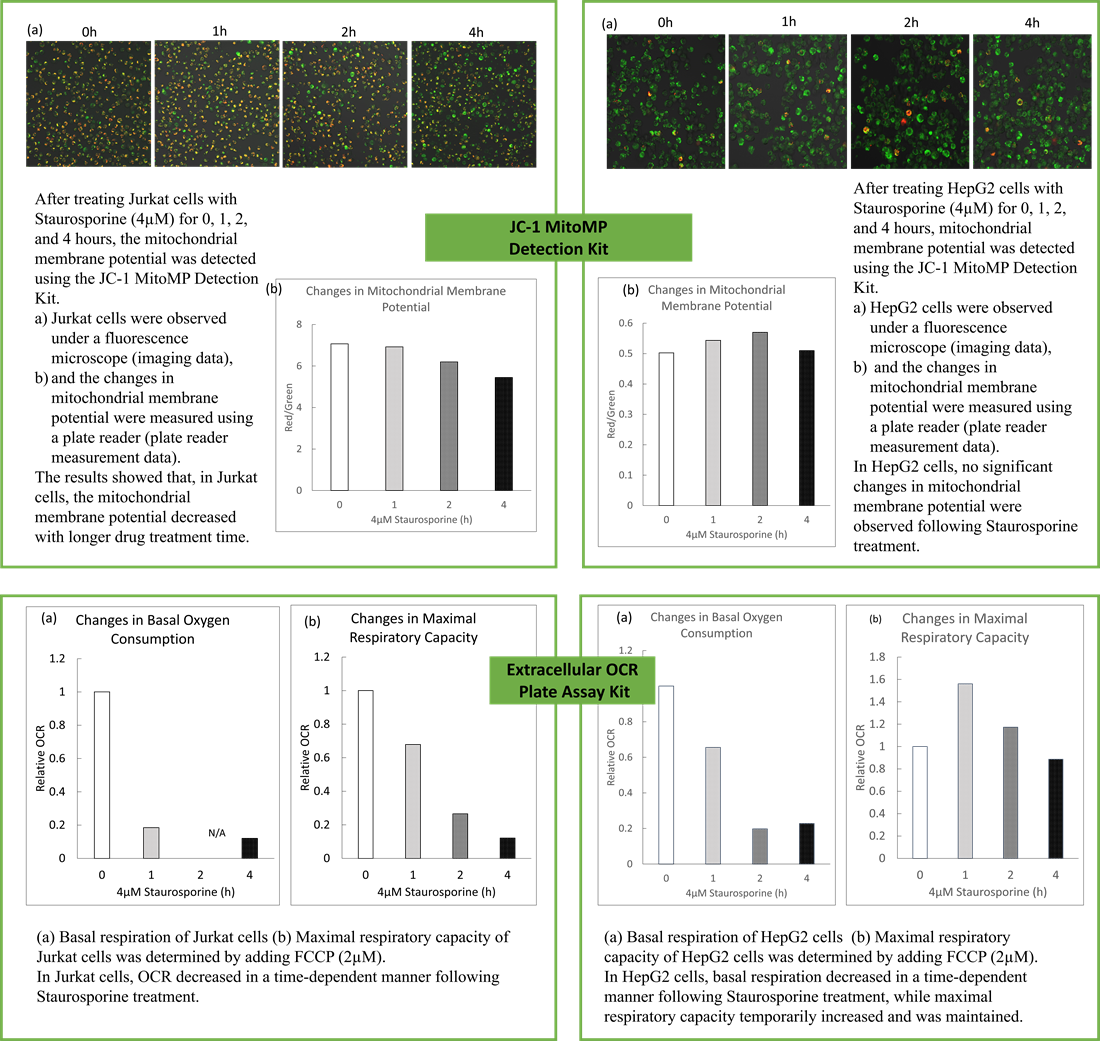
Products:
Glycolysys/OXPHOS - G270 Glycolysis/OXPHOS Assay Kit
OCR - E297 Extracellular OCR Plate Assay Kit
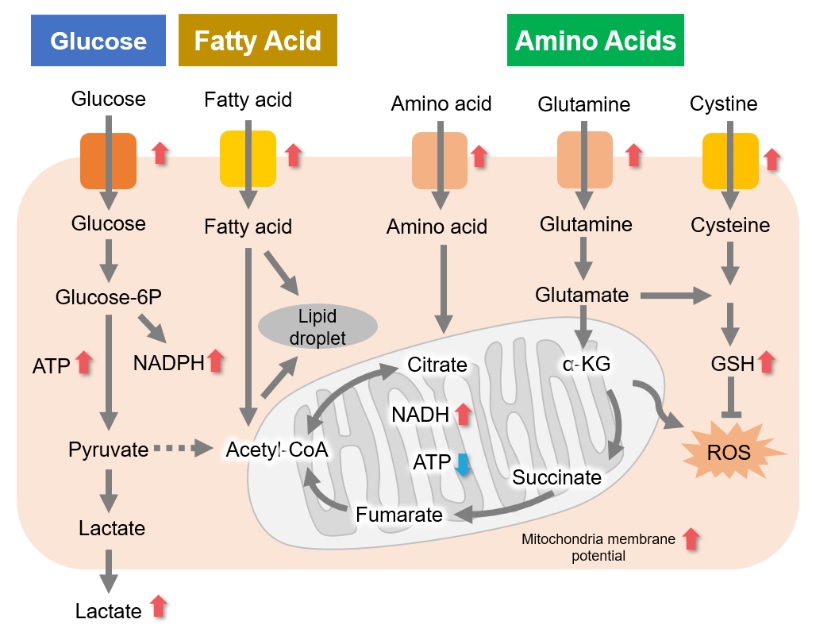
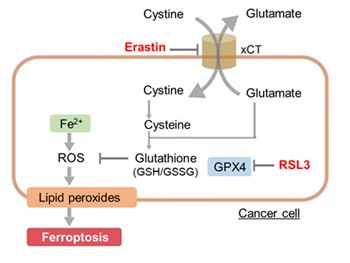
Many cancer cells produce ATP through the glycolytic pathway. On the other hand, it has been recently reported that cancer cells whose glycolytic pathway is suppressed survive by shifting their energy metabolism to OXPHOS by enhancing mitochondrial function, and the dependency of metabolic pathways differs depending on cell lines.
The dependence of OXPHOS and Glycolysis in two types of cancer cells, HeLa and HepG2, were compared based on Lactate production, ATP levels, and OCR values.
<Evaluation by Lactate production and ATP levels>
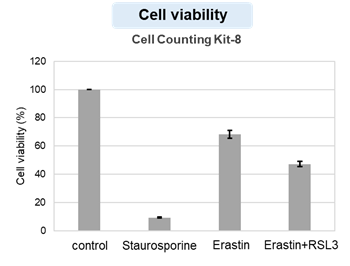
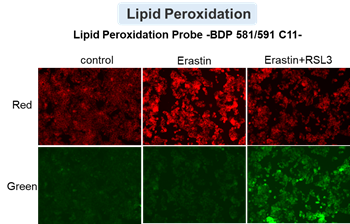
We confirmed the changes in ATP and Lactate production when ATP synthesis by OXPHOS was inhibited by Oligomycin stimulation and by 2-Deoxy-D-glucose (2-DG) in the glycolytic pathway. The results showed that HeLa cells depend on Glycolysis and HepG2 cells depend on OXPHOS to synthesize ATP.
*Please refer to the "Supplementary information on technology and products used" section on the right for additional information on the results.
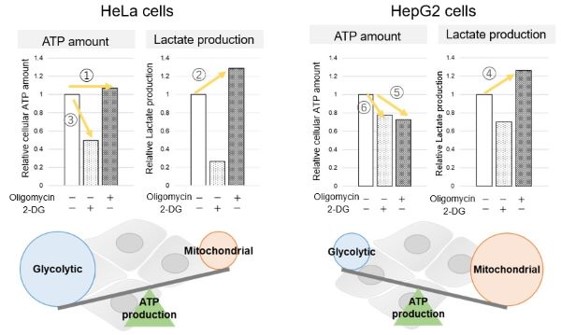
-
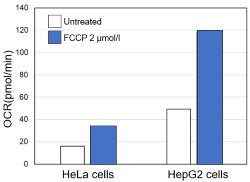
<Evaluation by OCR value>
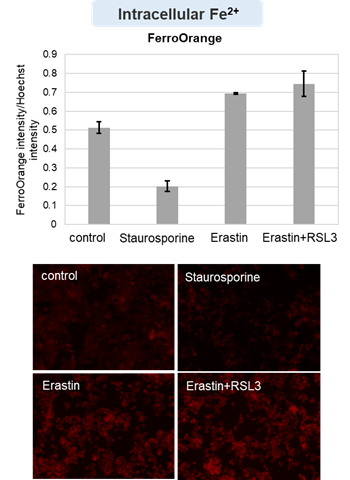
Using the same number of cells, we measured the OCR value when cellular oxygen consumption was promoted by stimulating the cells with FCCP, a mitochondrial uncoupling agent. The results showed that HepG2 cells had higher OCR values than HeLa cells, suggesting a greater dependence on OXPHOS, correlating with the results obtained from ATP level and Lactate production.