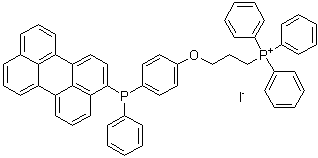
MitoBright LT Green
Mitochondrial Staining
- High Intracellular Retention
- Long-Term Visualization of Mitochondria
- Provided as Ready-to-Use DMSO Solution
- Allows for Collagen-coated Plates
-
Product codeMT10 MitoBright LT Green
| Unit size | Price | Item Code |
|---|---|---|
| 400 ul | Find your distributors | MT10-12 |
Description
A mitochondrion is a multifunctional organelle not only involved in energy production in a cell, but other additional cellular functions. The active cycle of mitochondrial fusion and division induces morphological changes, which is called mitochondrial dynamics. Abnormalities in morphological control of mitochondria are associated with neurodegenerative diseases, metabolic disorders, aging, and so on. Therefore, the demand for long-term observation of mitochondrial dynamics has recently been increasing.
Methods for monitoring mitochondrial morphology, dynamics, and number are usually based on small fluorescent molecules or plasmid transfection techniques. The use of plasmids requires the target protein to be stably expressed, while small fluorescent molecules are widely used because they can simply be added to cells. Among commercially available small fluorescent molecules, those containing the chloromethyl moiety are commonly used. However, these dyes have some limitations, including short-term retention in cells, decreased fluorescence intensity in serum, and high background.
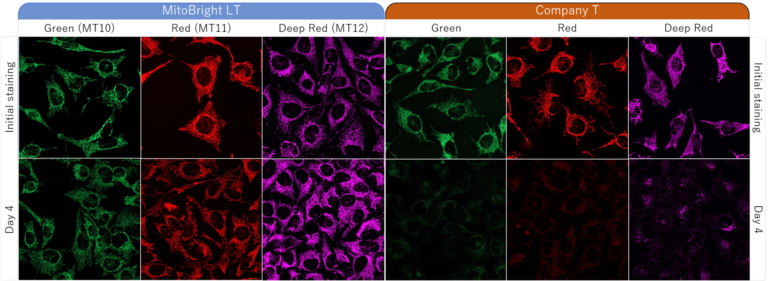
Dojindo’s MitoBright LT dyes overcome these limitations. MitoBright LT dyes are designed to exhibit mitochondria retention for long-term visualization. In addition, the MitoBright LT dyes show stronger fluorescence signals compared with other commercially available dyes that contain the chloromethyl moiety. The MitoBright LT dyes offer three different color options (Green, Red and Deep Red), and are provided as a ready-to-use DMSO solution. A working solution can easily be prepared in a single dilution step with growth medium or HBSS.
Manual
Technical info
HeLa cells were washed with HBSS (Hank’s Balanced Salt Solution) and subsequently stained with each of MitoBright LTs or an existing reagent. The culture medium was replaced with serum-containing medium, and mitochondria were observed after 4 days of incubation. As a result, fluorescence intensity of an existing reagent decreased significantly after 4 days, but in MitoBright LT, fluorescence intensity remained unchanged and mitochondria were clearly observable. Moreover, after further incubation, we confirmed that MitoBright LT was retained in mitochondria even after 7 days.
Dojindo Laboratories' Long-term mitochondrial staining reagent MitoBright LT series is widely used around the world.
| Product Name | Samples | Observation time after staining |
Instrument | Publications |
|---|---|---|---|---|
| MitoBright LT Green |
Cell(C2C12) |
5 Days | Fluorescence microscope | Polymers |
| MitoBright LT Green | Cell(BMM) | 36 hrs or 3 Days | Fluorescence microscope/ Flow Cytometer |
JCI Insights |
| MitoBright LT Red | Cell(A11, P29) | 3 Days | Fluorescence microscope | BMC Mol. Cell Biol. |
| MitoBright LT Deep Red | Cell(HT-1080, MCF-10A, MCF-7 ) | 8 hrs | Fluorescence microscope | Advanced Therapeutics |
| MitoBright LT Deep Red | Cell(4T1) | 24 hrs | Fluorescence microscope | Advanced Functional Materials |
Stained in serum-contained media
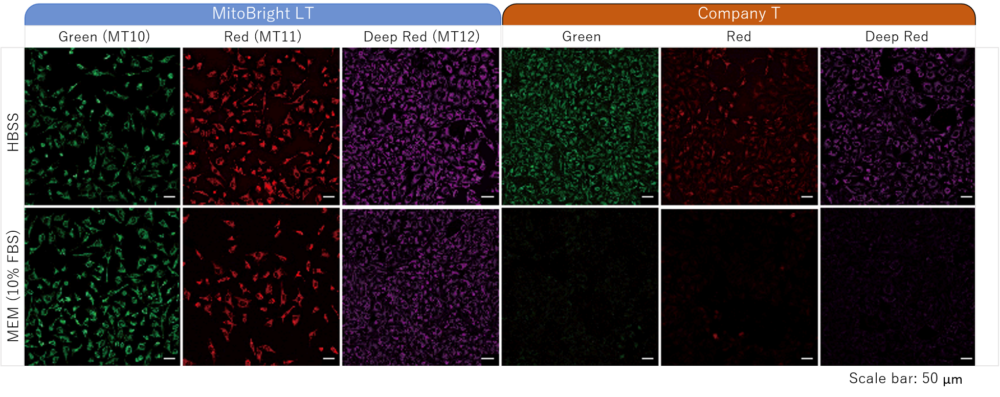
Staining was performed with either MitoBright LT or an existing reagent in serum-containing or serum-free medium. In serum-containing medium, low fluorescence intensity was observed when using the existing reagent, however the cells stained with MitoBright LT experienced clear, resilient fluorescence of mitochondria
Fixed cell imaging
1. HeLa cells were seeded on a μ-slide 8 well plate and cultured at 37°C overnight in a 5% CO2 incubator.
2. The supernatant was removed and the cells were washed with serum-containing medium twice.
3. 0.1 μmol/l MitoBright LT (Green/Red/Deep Red) working solution (serum-containing medium) was added and the cells were incubated at 37°C for 15-60 min in the 5% CO incubator.
4. The supernatant was removed and the cells were washed with PBS twice.
5. 4% paraformaldehyde (PFA) solution was added and the cells were fixed at room temperature for 15 minutes.
6. The supernatant was removed and the cells were washed with PBS twice.
7. The cells were observed under a fluorescence microscope.
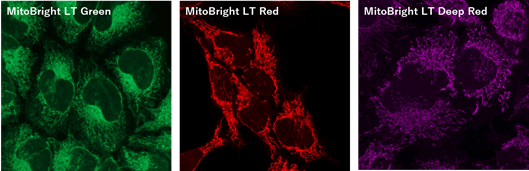
Figure 1. Fluorescent images of 4% PFA fixed HeLa cells (Stain then Fix)
Note: Cells should be fixed with paraformaldehyde (PFA). Fixation with methanol or other solvents extracts lipids and results in poor staining.
Note: The staining has low tolerance for permeabilization after fixation, and cannot be used with detergent.
(Triton X-100, NP-40 etc.)
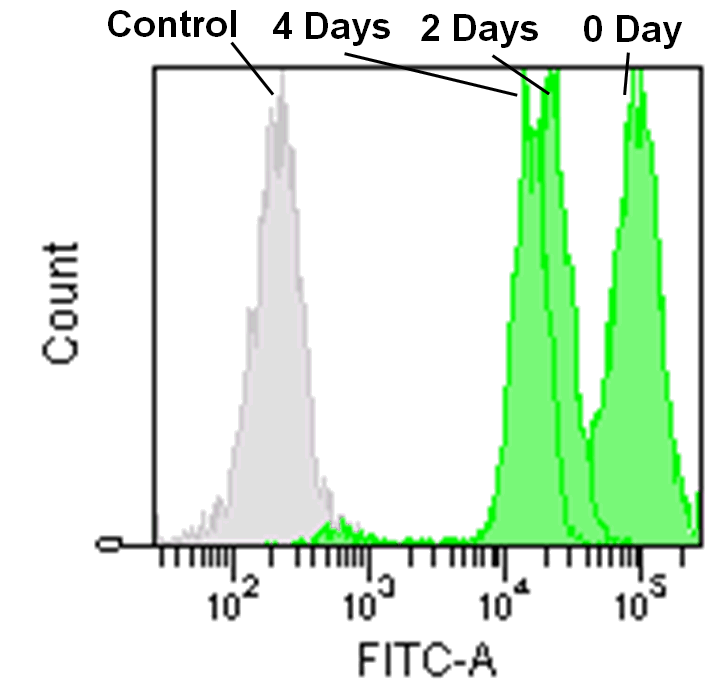
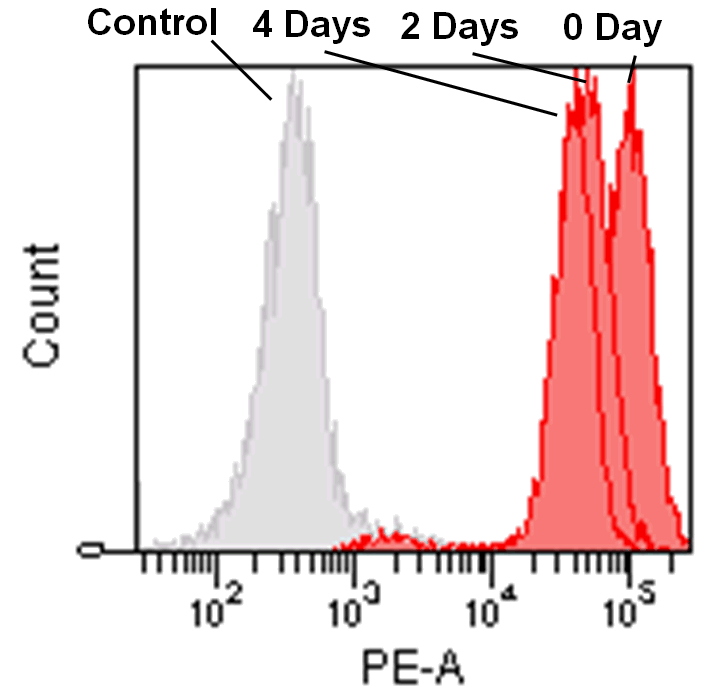
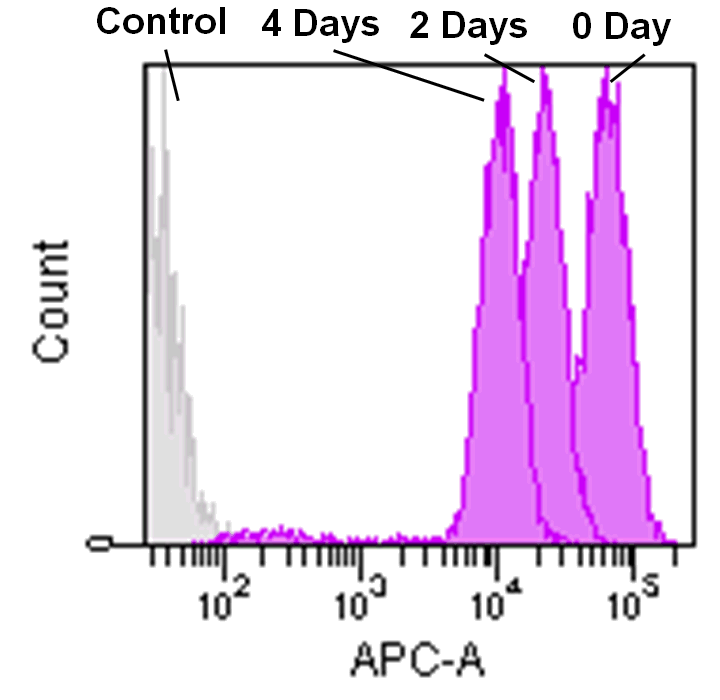
Detection by flow cytometry
Jurkat cells (3.2×105 cells/mL) were suspended in RPMI medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin, plated on a 35cm dish, and incubated overnight at 37˚C in a 5% CO2 atmosphere. RPMI medium was removed and replaced with 5mL of MitoBright LT working solution (0.1μmol/L). The cells were then incubated for 30 minutes at 37˚C. The working solution was removed and the cells were washed twice with 5mL of RPMI medium. Fresh RPMI medium was poured into the dish and cells were passaged and analyzed every 2 days by flow cytometry.
Detection of mitochondria using a collagen coated tissue plate.
Collagen-coated tissue culture plates are often used for examination of mitochondrial morphology because this imaging requires high magnifications.
The existing mitochondrial staining reagent bound to collagen, resulting with an increase in background staining, whereas the MitoBright LT series yielded clearly stained mitochondria.
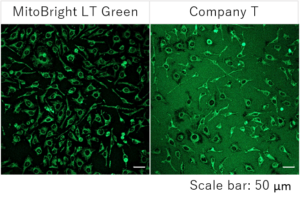
Conditions:
Staining: HeLa cells were plated on a collagen coated tissue culture plate and cultured for 24 hours. The culture medium was removed, and the cells were washed with HBSS.
MitoBright LT Green working solution (100 nmol/L) was poured into the plate, the cells were incubated for 30 minutes. The solution was removed, and the cells were washed with HBSS. A fluorescence microscope was used for imaging of mitochondria.
Detection: Ex: 488 nm, Em: 500-560 nm
Result:
In mitochondria stained with an existing reagent, background staining was observed, but the mitochondria stained with MitoBright LT were clearly stained without having effect of background staining.
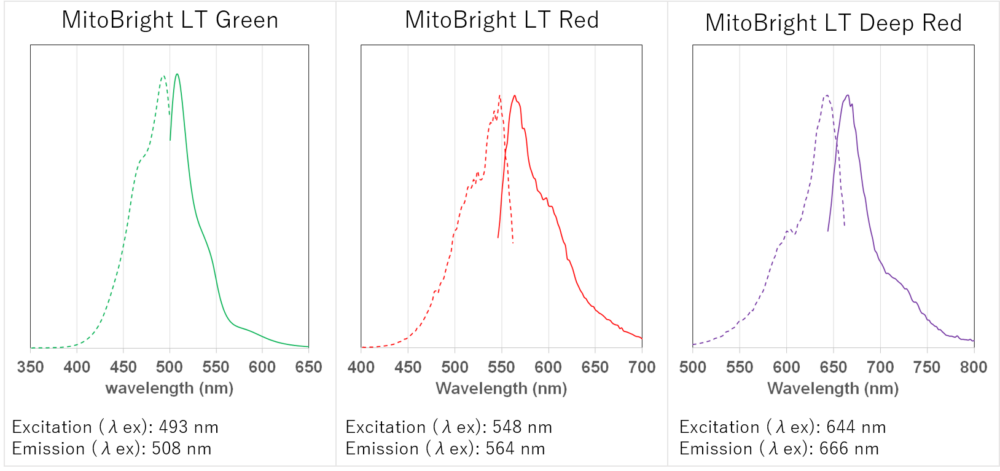
Fluorescence Properties
References
| No. | Sample | Instrument | Reference(Line) |
|---|---|---|---|
| 1 | Cell (U251) | Microscope | Y, Shinoda, K. Kujirai, K. Aoki, M. Morita, M. Masuda, L. Zhang, Z. Kaixin, A. Nomoto, T. Takahashi, Y. Tsuneoka, J. Akimoto, H. Kataoka, R. Rachi, A. Narumi, T. Yoshimura, S. Yano and Y. Fujiwara, ”Novel Photosensitizer β-Mannose-Conjugated Chlorin e6 as a Potent Anticancer Agent for Human Glioblastoma U251 Cells.", Pharmaceuticals., 2020, 13(10), 316. |
| 2 | Yeast (Lipomyces starkeyi) | Microscope | L. Duan and K. Okamoto, "Mitochondrial dynamics and degradation in the oleaginous yeast Lipomyces starkeyi", Genes Cells., 2021, 10.1111/gtc.12875 . |
| 3 | Cell (HeLa) | Plate Reader | W. Islam, Y. Matsumoto, J. Fang, A. Harada, T. Niidome, K. Ono, H. Tsutsuki, T. Sawa, T. Imamura, K. Sakurai, N. Fukumitsu, H. Yamamoto, H. Maeda., "Polymer-conjugated glucosamine complexed with boric acid shows tumor-selective accumulation and simultaneous inhibition of glycolysis ", Biomaterials., 2021, 269, (-), 120631. |
| 4 | Cell (Naive CD4+ T cells) | Fluorescence microscope | T. Kanno, T. Nakajima, Y. Kawashima, S. Yokoyama, H. K. Asou, S. Sasamoto, K. Hayashizaki, Y. Kinjo, O. Ohara, T. Nakayama and Y. Endo, "Acsbg1-dependent mitochondrial fitness is a metabolic checkpoint for tissue Treg cell homeostasis", 2021, doi:10.1016/j.celrep.2021.109921. |
| 5 | Cell (CT26) | Flow Cytometer | L. Sun, K. Morikawa, Y. Sogo and Y. Suiura, "MHY1485 enhances X-irradiation-induced apoptosis and senescence in tumor cells", 2021, doi:10.1093/jrr/rrab057. |
| 6 | Cell (C2C12) | Fluorescence microscope | C. Lin, P. Wu, K. Liu, Y. Fan and J. Yu, "An Environmental Friendly Tapioca Starch-Alginate Cultured Scaffold as Biomimetic Muscle Tissue", 2021, doi:10.3390/polym13172882. |
| 7 | Cell (BMM) | Fluorescence microscope/ Flow Cytometer |
W. Ling, K. Krager, K. K. Richardson, A. D. Warren, F. Ponte, N. Aykin-Burns, S. C. Manolagas, M. Almeida and H. Kim, "Mitochondrial Sirt3 contributes to the bone loss caused by aging or estrogen deficiency", 2021, doi:10.1172/jci.insight.146728. |
| 8 | Cells (mouse erythrocytes) | Fluorescence microscope | M. Niikura, T. Fukutomi, J. Mitobe and F. Kobayashi, "Roles and Cellular Localization of GBP2 and NAB2 During the Blood Stage of Malaria Parasites", 2021, doi:10.3389/fcimb.2021.737457. |
Q & A
-
Q
Does MitoBright LT Green in DMSO solution not degrade after repeated freeze-thaw cycles?
-
A
We have confirmed that staining was possible using a solution that was subjected to 30 cycles of freeze-thaw cycles.
-
Q
Does depolarization after MitoBright LT staining affect fluorescent intensity?
-
A
Effects are confirmed by depolarization conditions and cell type. Also, the degree of influence differs for each MitoBright LT dye.
For reference, we stained HeLa cells with each MitoBright LT dye and observed changes in fluorescent staining after depolarization under the following conditions.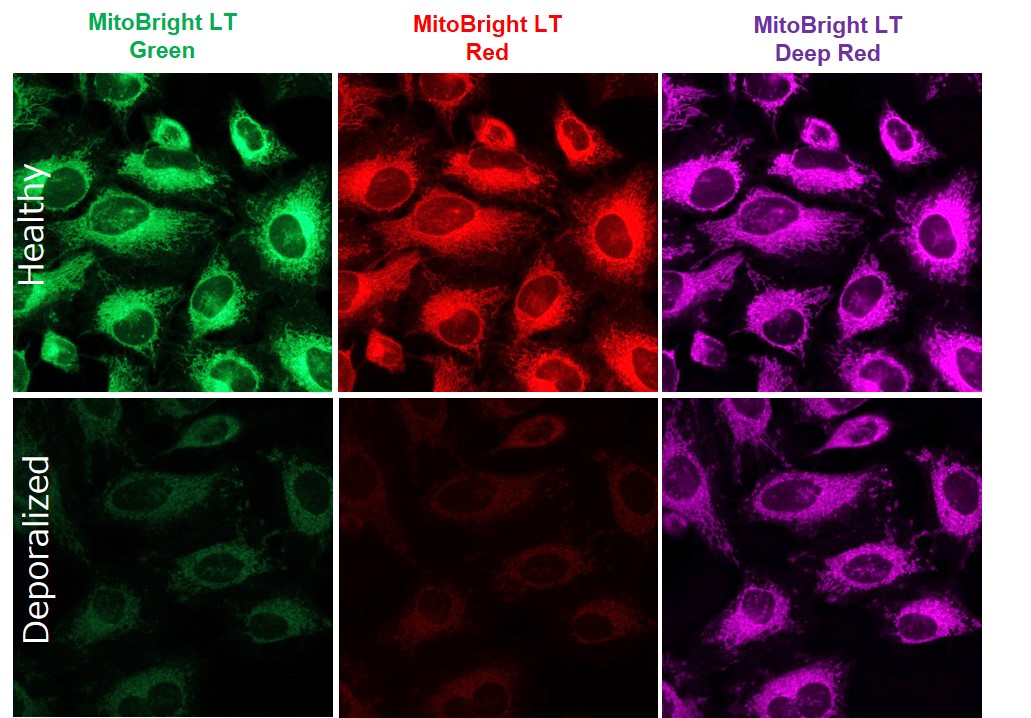
<Staining conditions>
HeLa cells were stained with MitoBright LT dyes (100 μmol/l, 30 min incubation), washed with HBSS, treated with FCCP (100 μmol/l, 60 min incubation), washed twice with HBSS, and observed.<Detection conditions>
MitoBright LT Green: Ex 488 nm/Em 500-560 nm
MitoBright LT Red: Ex 561 nm/Em 560-620 nm
MitoBright LT Deep Red: Ex 640 nm/Em 650-700 nm
Handling and storage condition
| Appearance: | Yellow liquid |
|---|---|
| Absorbance: | 0.600~0.800 |
| -20°C | |
|
Danger / harmful symbol mark |

|
|---|---|