PlasMem Bright Green
Cell Membrane Staining
- Applicable to live cells and fixation after staining
- High retentivity of reagents with low toxicity
- Just add reagents into medium
-
Product codeP504 PlasMem Bright Green
| Unit size | Price | Item Code |
|---|---|---|
| 100 ul | $288.00 | P504-10 |
General number of usable assays per 100 μl
35 mm dish x 10
μ-Slide 8 well x 10
Description
Detection Principle
The plasma membrane (PM), consists of a lipid bilayer separating the intracellular environment from the extracellular space. Consequently, the PM plays a central role in many cell behaviors, such as cell migration, cell stretching, and signaling cascades. Additionally, PM dysfunction is an important biomarker because it is related to the cell status and is linked to many diseases.
Dojindo’s PlasMem Bright dyes overcome these limitations. PlasMem Bright dyes are designed to stain PMs for over a day. Furthermore, the PlasMem Bright dyes are more water-soluble compared with other commercially available dyes and can be diluted with culture medium. The PlasMem Bright dyes offer two different color options (green and red) and are provided as ready-to-use DMSO solutions. A working solution can be prepared easily via a single dilution step using growth medium or HBSS.
Manual
Technical info
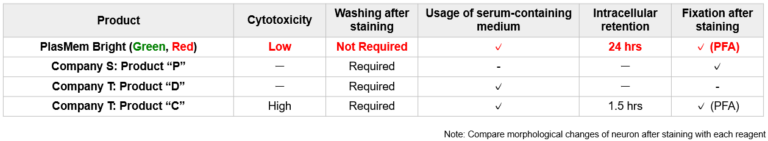
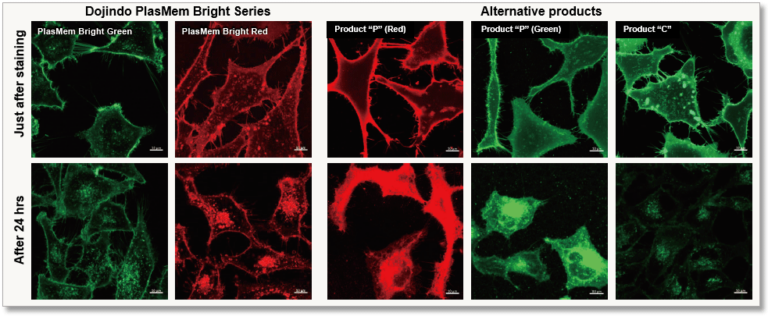
High retentivity on plasma membrane
HeLa cells stained with each plasma membrane staining reagent were incubated for 24 hrs and each the resulting fluorescent image was compared. PlasMem Bright series had higher retentivity on plasma membrane than other products.
Application Data: Cell membrane staining inside an ES cell colony
Mouse ES cells were cultured in a gelatin-coated glass bottom dish for 4 days, and the colonies were stained with PlasMem Bright Green (200x dilution) for 15 minutes and observed under a confocal microscope (Zeiss: LSM710) after medium exchange.
As a result, the membranes of the cells inside the colonies could be visualized with PlasMem Bright Green.

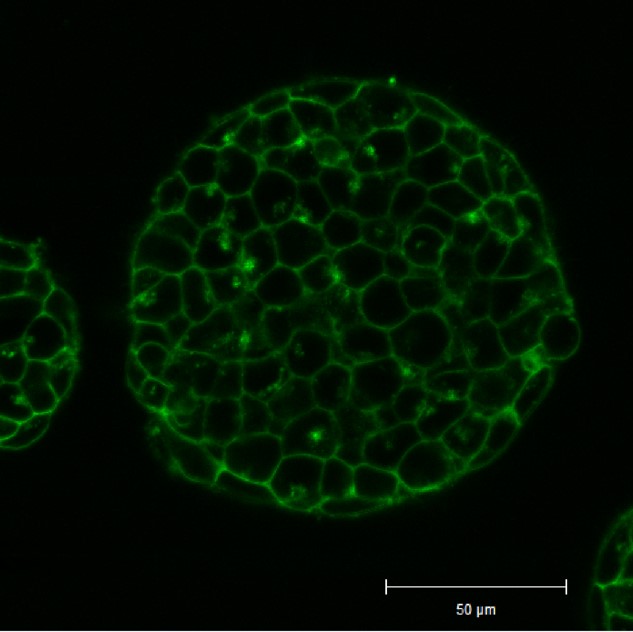
Cell Membrane(PlasMem Bright Green): Ex. 488 nm / Em. 500 - 560 nm
*The data is kindly provided by Dr. Hirotsugu Ishizu, Keio University School of Medicine.
Clear visualization of plasma membrane
Observe morphology of neuron (differentiated SH-SY5Y cells) and localization of mitochondria in axon.
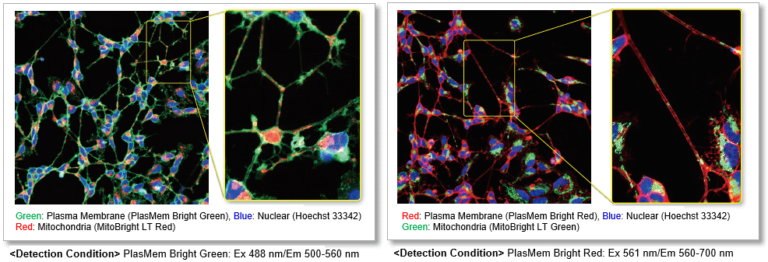
Application Data: Mitochondrial detection in neuroblast (SH-SY5Y cells)
The neuroblasts SH-SY5Y cells were stained with PlasMem Bright Green (green), MitoBright LT Red (red) and Hoechst 33342 (blue), and 3D images were obtained with a confocal fluorescence microscope.
Detection condition
Plasma Membrane (PlasMem Bright Green, green): Ex. 488 nm / Em. 500 – 560 nm
Mitochondria (MitoBright LT Red, red): Ex. 561 nm / Em. 560 – 620 nm
Nuclear (Hoechst 33342, blue): Ex. 405 nm / Em. 400 – 450 nm
Protocol
(1) Wash SH-SY5Y cells with HBSS
(2) Add PlasMem Bright Green (diluted 200 times), Hoechst 33342 (final concentration: 5 µg / ml) and MitoBright LT Red (final concentration: 0.1 µmol / l) prepared in the medium.
(3) Incubate for 10 minutes
(4) Wash the cells twice with HBSS
(5) Observation with a fluorescence microscope
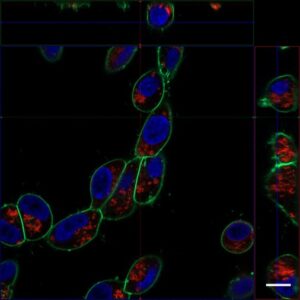
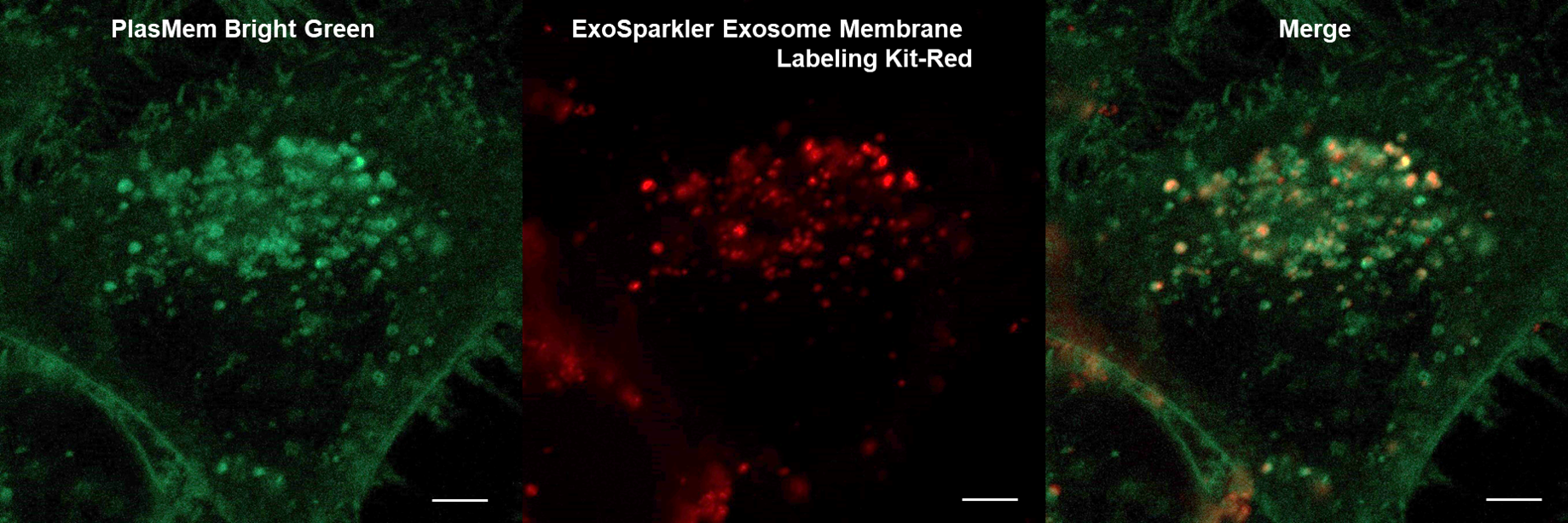
Application Data: Co-staining with exosomes
HeLa cells stained with PlasMem Bright Green were added with exosomes stained with the ExoSparkler Exosome Membrane labeling Kit-Red, and the uptake of exosomes into the cells was observed.
HeLa cells (Live cell)
HeLa cells (PFA fixed cells)
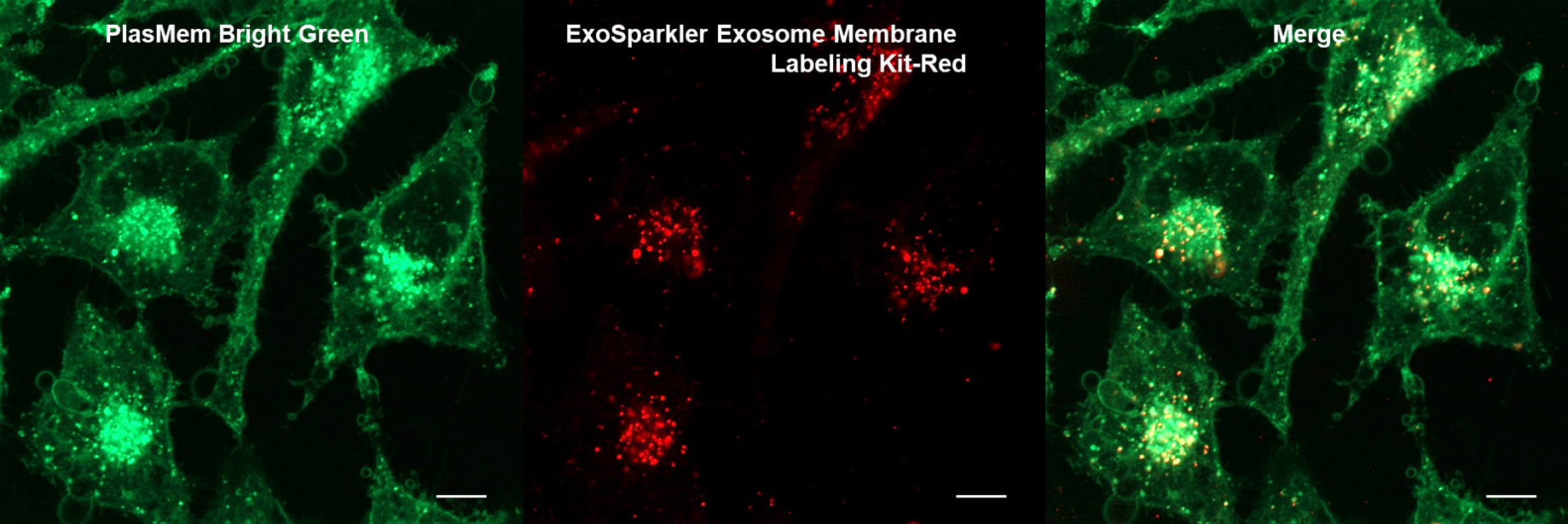
Plasma Membrane (PlasMem Bright Green, green): Ex. 488 nm / Em. 500 –560 nm
Exosome (ExoSparkler Exosome Membrane Labeling Kit-Red, red): Ex. 561 nm / Em. 560 –620 nm
(1) HeLa cells and incubate for 24 hours
(2) Remove the supernatant and add PlasMem Bright Green (100-fold dilution) prepared in the medium.
(3) Incubate for 10 minutes
(4) Wash the cells 3 times with HBSS
(5) Add 175 μl of MEM medium and 25 ul of Exosome solution stained with ExoSparkler Exosome Membrane Labeling Kit-Red.
(6) Incubate overnight in a CO2 incubator
(For immobilization) Wash cells twice with HBSS, add 4% PFA, incubate for 15 minutes, then wash cells twice with HBSS
(7) Observed with a confocal laser scanning microscope
Application Data: Time-lapse imaging of brain-derived mouse neuroblastoma (N1E-115 cells)
Time-lapse imaging of N1E-115 cells stained with PlasMem Bright Green diluted 200-fold in medium for 30 min.
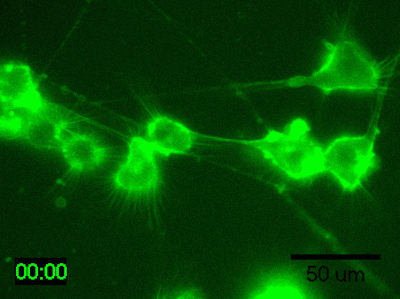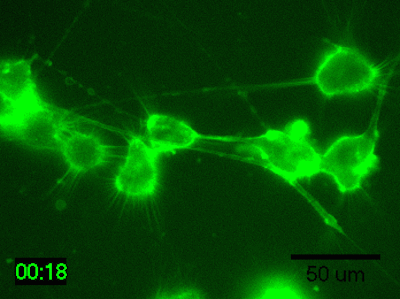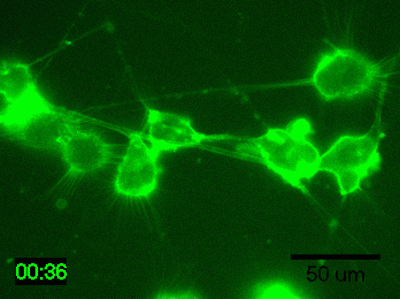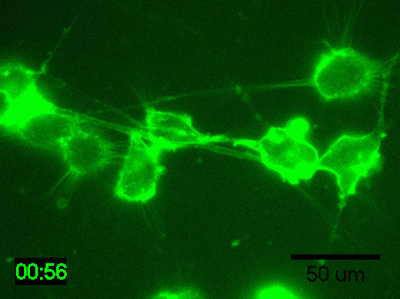
Detection conditions
・ Cells: N1E-115 cells (brain-derived mouse neuroblastoma)
・ Medium: 5% FBS, 1% glutamine-containing D-MEM (Low-glucose)
・ Culture equipment: 35 mm glass bottom dish
・ Imaging equipment: Fluorescence microscope with incubator
・ Shooting time: 1 hour, shooting interval: 2 minutes
Data was kindly provided from Dr. K Fukui, Shibaura Institute of Technology.
Application Data: Staining of tobacoo cultured BY-2 cell
BY-2 tobacco cells were stained with PlasMem Bright Green (200-fold dilution) for 5 minutes and washed, and then, long-term observation was performed every 5 minutes. The cell membrane structure and the formation of the cell plate during the cytokinesis were clearly observed.
(Kindly provided by Dr. Daisuke Kurihara, Nagoya University)
・Observed with spinning disk confocal microscope.
・Ex: 488 nm; Em: 520/35 (502.5-537.5 nm)
・Time in this video is expressed as 00:00(hour: minute).
Applicaiton Data: Staining of Arabidopsis thaliana roots
The roots were stained with PlasMem Bright Green (200x dilution) for 15 minutes and then observed for fluorescence by z-stack photography. From the acquired 3D fluorescence images, it was confirmed that each cell membrane was fluorescently detected.
(Kindly provided by Dr. Daisuke Kurihara, Nagoya University)
・Observed with Spin disk confocal microscope, Z-stack
・Ex: 488 nm; Em: 520/35 (502.5-537.5 nm)
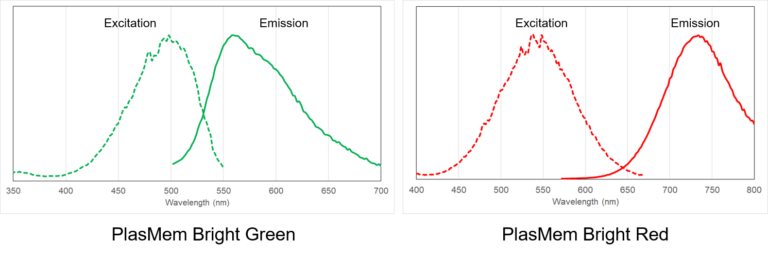
Excitation and emission spectra of PlasMem Bright dyes
References
| No. | Sample | Instrument | Reference (Link) |
|---|---|---|---|
| 1) | Cell (CML Cell) |
Fluorescence microscope | T. Hoshiko, Y. Kubota, R. Onodera, T. Higashi, M. Ykoo, K. Motoyama and S. Kimura, "Folic Acid-Appended Hydroxypropyl- -Cyclodextrin Exhibits Potent Antitumor Activity in Chronic Myeloid Leukemia Cells via Autophagic Cell Death", Cancers, 2021, doi:10.3390/cancers13215413. |
| 2) | Cell (HCT116 Cell) |
Fluorescence microscope | Z. Liu, Y. Li, Y. Zhu, N. Li, W. Li, C. Shang, G. Song, S. Li, J. Cong, T. Li, Z. Xiu, J. Lu, C. Ge, X. Yang, Y. Li, L. Sun, X. Li and N. Jin, "Apoptin induces pyroptosis of colorectal cancer cells via the GSDME-dependent pathway", Int. J. Biol. Sci., 2022, doi:10.7150/ijbs.64350. |
| 3) |
Cell |
Fluorescence microscope | L.Haisha, M. Xinyu, Z. Yuanyuan, L. Yuheng, L. Nan, Z. Weiying, C. Jianhui, L. Boqi, D. Wanqing, L. Xiaohui, Y. Li, "The formation of migrasomes is initiated by the assembly of sphingomyelin synthase 2 foci at the leading edge of migrating cells", Nature cell biology, 2023, doi: 10.1038/s41556-023-01188-8. |
Q & A
-
Q
Can I fix the cells?
-
A
Fix cells with 4% PFA is possible.
However, do not use cell permeabilization reagents which may cause loss of fluorescence.
-
Q
Is it possible for long-term fluorescent observation?
-
A
It is possible.
However, please try to keep the excitation to the minimum amount. Exposure of cells to excitation light may cause cell damage and degradation of the dye, optimizing the factors such as interval time.
-
Q
When preparing the working solution, is it possible to dilute it with serum-free medium or buffer?
-
A
Yes, it is possible to prepare the working solution with serum-free medium or buffer.
-
Q
What should I use to wash the cells after staining?
-
A
Serum-free medium or buffers like PBS and HBSS can be used for washing the cells after staining.
-
Q
After adding the working solution, is it possible to observe the fluorescence immediately?
-
A
Yes, it is possible to immediately observe the fluorescence after adding the working solution. According to the protocol, it is recommended to add the reagent and incubate for 5 minutes before observation.
-
Q
It seems like some place other than cell membrane has been stained. What is the possible reasons?
-
A
There are two possible reasons:
1 Some of the reagents retained in the cell membrane are taken up into the cell by endocytosis over time.
(Please refer to the figure in "Technical Info: High retentivity on plasma membrane" on this page.)2 If the focus is at the bottom of the cells (adhesive surface) the image may be observed as Fig. A.
Please try to adjust the height of the Z-axis as shown in Fig. B
-1.jpg)
-2.jpg)
-
Q
Can I co-culture the cells stained with PlasMem Bright Green and the cells stained with PlasMem Bright Red to tell them apart?
-
A
When the cells come into contact with each other, the PlasMem dye moves to the other cells, so it cannot be told apart.
Handling and storage condition
| Appearance: | Orange liquid |
|---|---|
| Dye content: | To pass test |
| -20°C | |
|
Danger / harmful symbol mark |

|
|---|---|