Fura 2
Reagent for Monitoring Intracellular Calcium Ion
-
Product codeF014 Fura 2
-
CAS No.96314-98-6
-
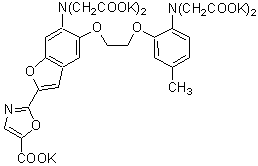
Chemical name1-[6-Amino-2-(5-carboxy-2-oxazolyl)-5-benzofuranyloxy]-2-(2-amino-5-methylphenoxy)ethane-N,N,N',N'-tetraacetic acid, pentapotassium salt
-
MWC29H22K5N3O14=831.99
| Unit size | Price | Item Code |
|---|---|---|
| 1 mg | $351.00 | F014-10 |
Product Description
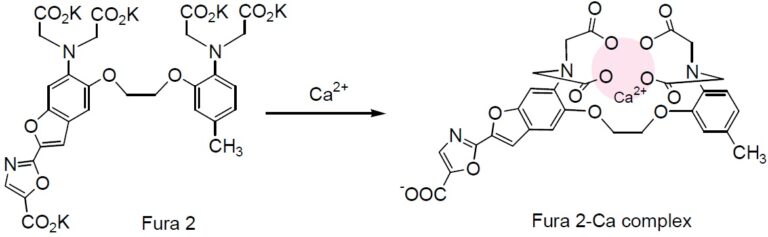
Fura 2 was developed to improve the fluorescent properties of Quin 2. The signal intensity in 1 mM of loaded Fura 2 corresponds to that of 30 mM of loaded Quin 2. This allows an experiment at a lower concentration of indicator using Fura 2 as compared to Quin 2. Fura 2 is one of the most widely used calcium indicators for ratiometric measurement. Many types of instrumentation are now available for experiments using Fura 2. Fura 2 is especially suitable for digital imaging microscopy. It is less susceptible to photobleaching than Indo 1. Changes in the cell shape can sometimes affect the fluorescent ratio at 340 nm and 380 nm. For example, fluorescent signal intensities at these wavelengths sometimes decrease simultaneously with smooth muscle contraction. For blood vessels, however, the increase of the signal intensity at 340 nm tends to be smaller on contraction, while the decrease of the signal intensity at 380 nm tends to be larger with its contraction. Fura 2-AM is an acetoxymethyl ester derivative of Fura 2 that can be easily loaded into cells by incubation.
Chemical Structure
Technical info
General Protocol (for NG 108-15/ Neuronal Cell Line)*
Reagents:
1 mM Fura 2-AM/DMSO (1 mg Fura 2-AM in 1 ml DMSO)
Hanks Ebalanced salt solution (HBSS)
HEPES buffer saline (20 mM HEPES, 115 mM NaCl, 5.4 mM KCl, 1.8 mM CaCl2, 0.8 mM MgCl2, 13.8 mM glucose, pH 7.4)
Protocol:
1. Culture cells on a glass-bottom dish using DMEM containing 5% fetal calf serum.
2. Change the medium to 1 mM dibutyl cAMP/DMEM, and culture the cells for 3-4 days to induce dendrites.
3. Dilute 1 mM Fura 2-AM DMSO solution with HEPES buffer saline to prepare 1 mM Fura 2-AM working solution.
4. Remove the culture medium, and add 0.5 ml of the Fura 2-AM working solution to the cells.
5. Incubate for 20 min. Then remove the Fura 2-AM working solution.
6. Wash the cells once with HEPES buffer saline. Then incubate the cells for 1 hour in the HEPES buffer saline.
7. Use the cells for fluorescent calcium ion detection.
8. Monitor the excitation spectra at 380 nm (calcium free) and 340 nm (calcium complex) with fixed emission at 510 nm.
*Cell staining conditions differ by cell types, so it is necessary to optimize the conditions for each experiment.
References
1. G. Grynkiewicz, et al., A New Generation of Ca2+ Indicators with Greatly Improved Fluorescence Properties. J Biol Chem. 1985;260:3440-3450.
2. D. A. Williams, et al., Calcium Gradients in Single Smooth Muscle Cells Revealed by the Digital Imaging Microscope Using Fura-2. Nature. 1985;318:558-561.
3. R. Y. Tsien, et al., Measurement of Cytosolic Free Ca2+ in Individual Small Cells Using Fluorescence Microscopy with Dual Excitation Wavelengths. Cell Calcium. 1985;6:145-157.
4. D. A. Williams, et al., Intracellular Calibration of the Fluorescent Calcium Indicator Fura-2. Cell Calcium. 1990;11:75-83.
5. W. Almers, et al., The Ca Signal from Fura-2 Loaded Mast Cells Depends Strongly on the Method of Dye-loading. FEBS Lett. 1985;192:13-18.
6. G. H. Rao, et al., Measurement of Ionized Calcium in Blood Platelets with a New Generation Calcium Indicator E Biochem Biophys Res Commun. 1985;132:652-657.
7. H. Ozaki, et al., Simultaneous Recordings of Calcium Signals and Mechanical Activity Using Fluorescent Dye Fura 2 in Isolated Strips of Vascular Smooth Muscle. Jpn J Pharmacol. 1987;45:429-433.
8. M, Mitsui, et al., Leakage of the Fluorescent Ca2+ Indicator Fura-2 in Smooth Muscle. Jpn J Pharmacol. 1993;61:165-170.
Handling and storage condition
| Appearance: | Yellow to orangish yellow powder or solid |
|---|---|
| Purity (HPLC): | ≧ 98.0% |
| Fluorescence spectrum: | To pass test |
| NMR spectrum: | Authentic |
| Ambient temperature |