Biofilm Formation Assay Kit

Biofilm Formation Assay
- Shorten measurement time significantly
- Reduce measurement variation
-
Product codeB601 Biofilm Formation Assay Kit
| Unit size | Price | Item Code |
|---|---|---|
| 96 tests | $208.00 | B601-10 |
| 96 tests | ・Crystal Violet Solution ・96-peg Lid ・96-well Plate |
22 ml ×1 ×1 ×10 |
|---|
Description
Biofilms are biological aggregates consisting of microorganisms and extracellular polysaccharides that can exist in many environments. The microorganisms in biofilms are highly resistant to antibiotics; therefore, research into medicines and food ingredients that have anti-biofilm activities is a growing area.
Our assay kits for the quantitative measurements of biofilm formation/inhibition of biofilm formation (Biofilm Formation Assay Kit) and anti-biofilm drug efficacy (Biofilm Viability Assay Kit) adopted a protocol which is designed to use the peg plate from biofilm formation. Our assay kits combined all the necessary components into each package.
Manual
Technical info
Feature 1: Measurement time can be significantly shortened
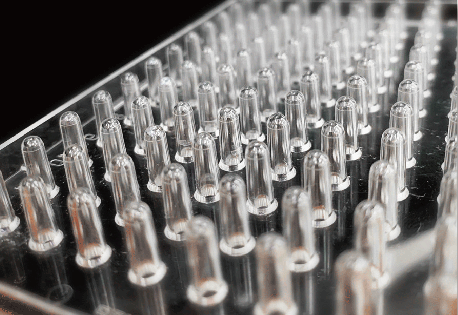
In existing methods, biofilms are formed on the bottom of the well of a microplate, and additional work is required to change microbial culture media and wash sample several times before and after staining. However, our assay kits allow you to form biofilms on the pegs attached to the plate’s lid. Media change and staining process can be done simply by transferring the lid; therefore, our assay kits are very easy to use.
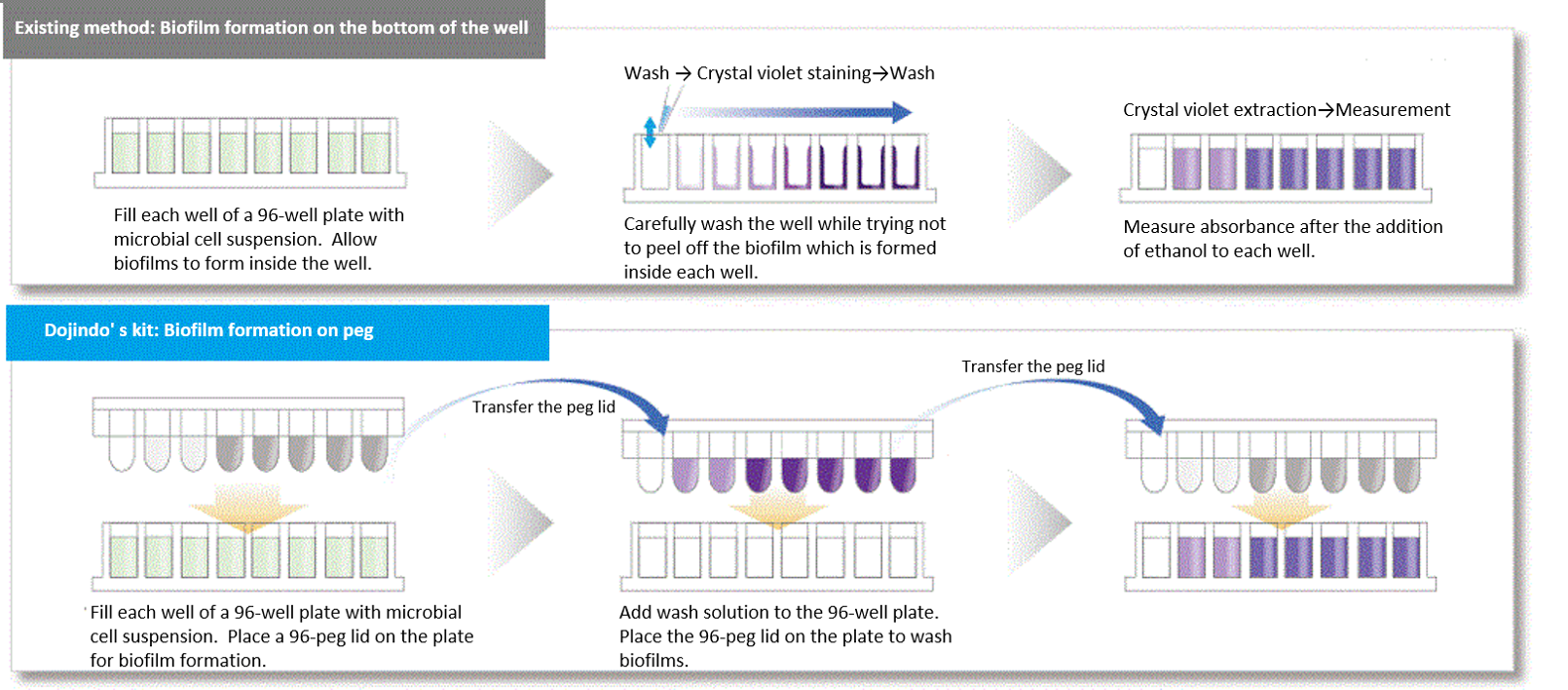
Feature 2: Our assay kits can reduce measurement variation.
In existing methods, biofilm formation occurs on the bottom of the well of a microplate, which made biofilm easier to peel off because of the processes such as washing. This causes measurement variation, and this measurement variation was an issue for the quantification of biofilm. Our assay kits allow you to form biofilms on the pegs attached to the plate’s lid and make it possible to avoid peeling off biofilm which occurs during the process of the formation.
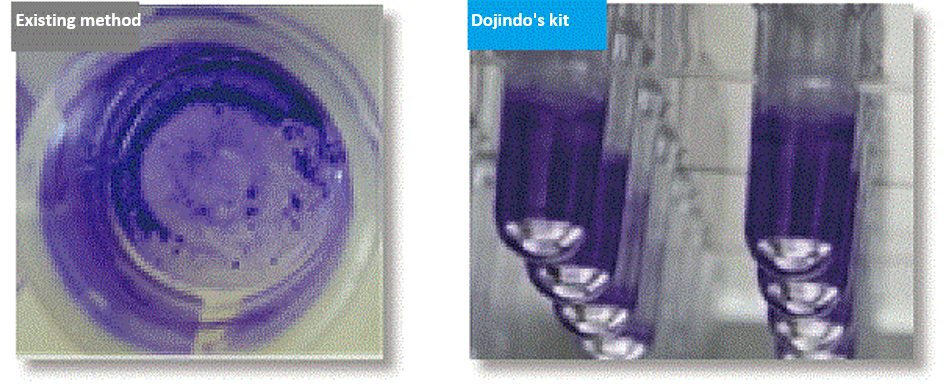
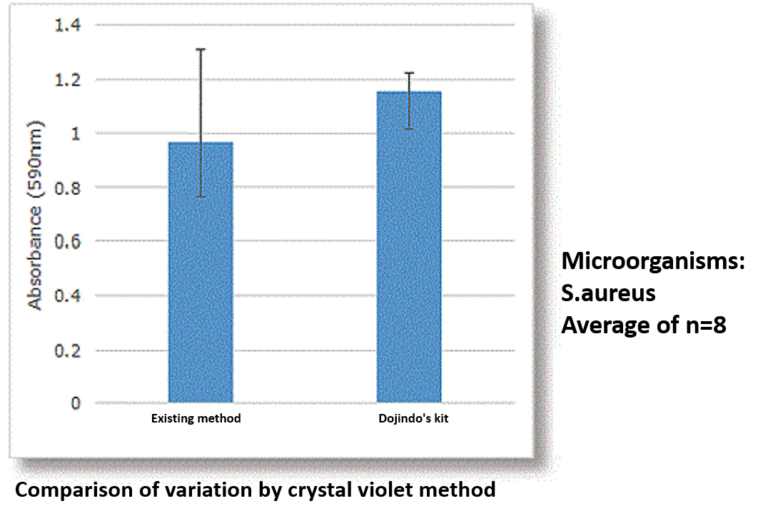
Reference (biofilm formation using 96-peg lid)
T. Tsukatani, F. Sakata, R. Kuroda, "A rapid and simple measurement method for biofilm formation inhibitory activity using 96‑pin microtiter plate lids", World J. Microbiol. Biotechnol., 2020, doi:10.1007/s11274-020-02964-6.
Two different types of assay kits for different purposes.
We offer two types of assay kits that measure the amount of biofilm formation or the metabolic activities of live microorganisms within biofilms by using the same measurement method. You can select the one that best suits your needs.
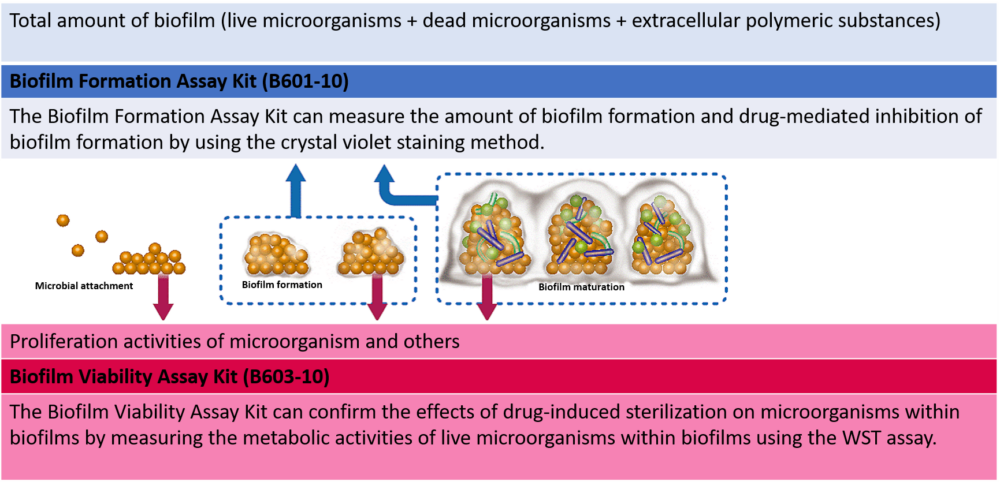
Selection of 2 types of assay kits
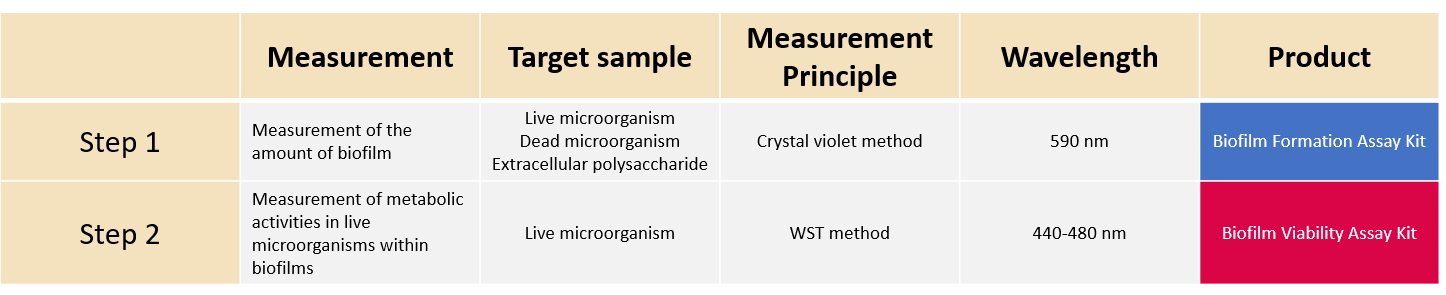
*Conditions on biofilm formation vary according to microbial species and strains. When you need to examine these conditions, we first recommend you use the Biofilm Formation Assay Kit.
*The Biofilm Formation Assay Kit is a product developed together with Fukuoka Industrial Technology Center.
Procedure
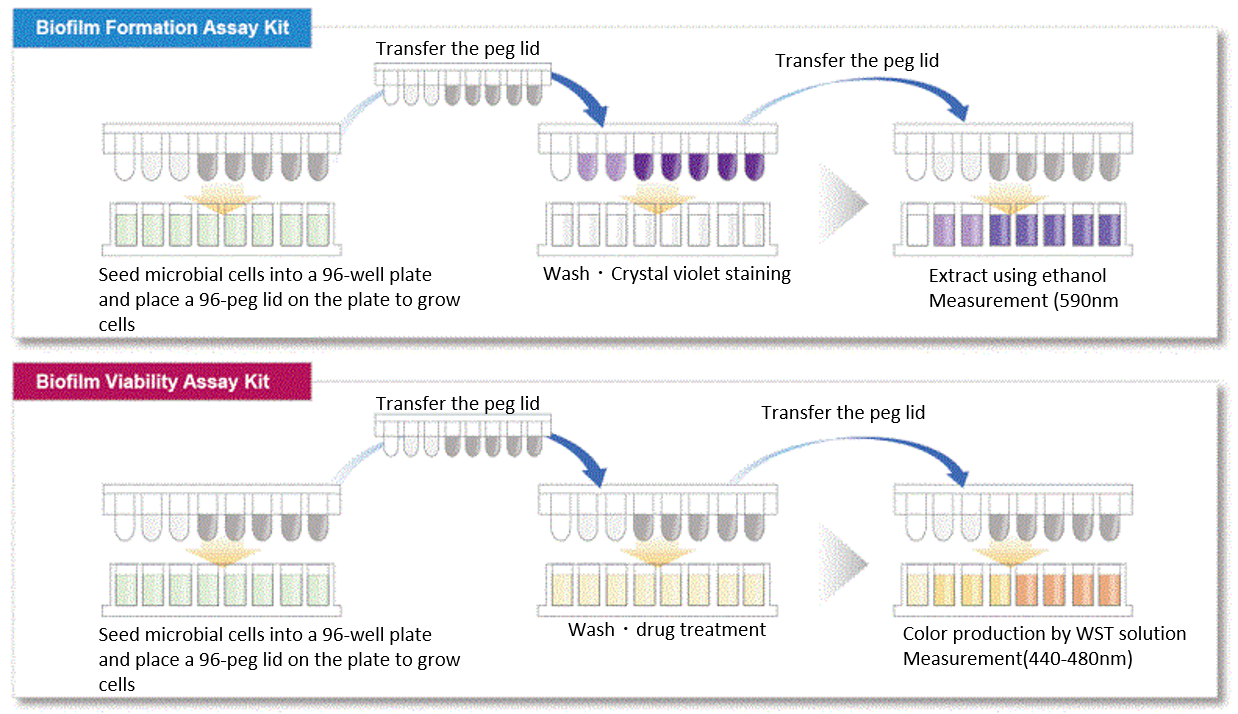
Example of measurement
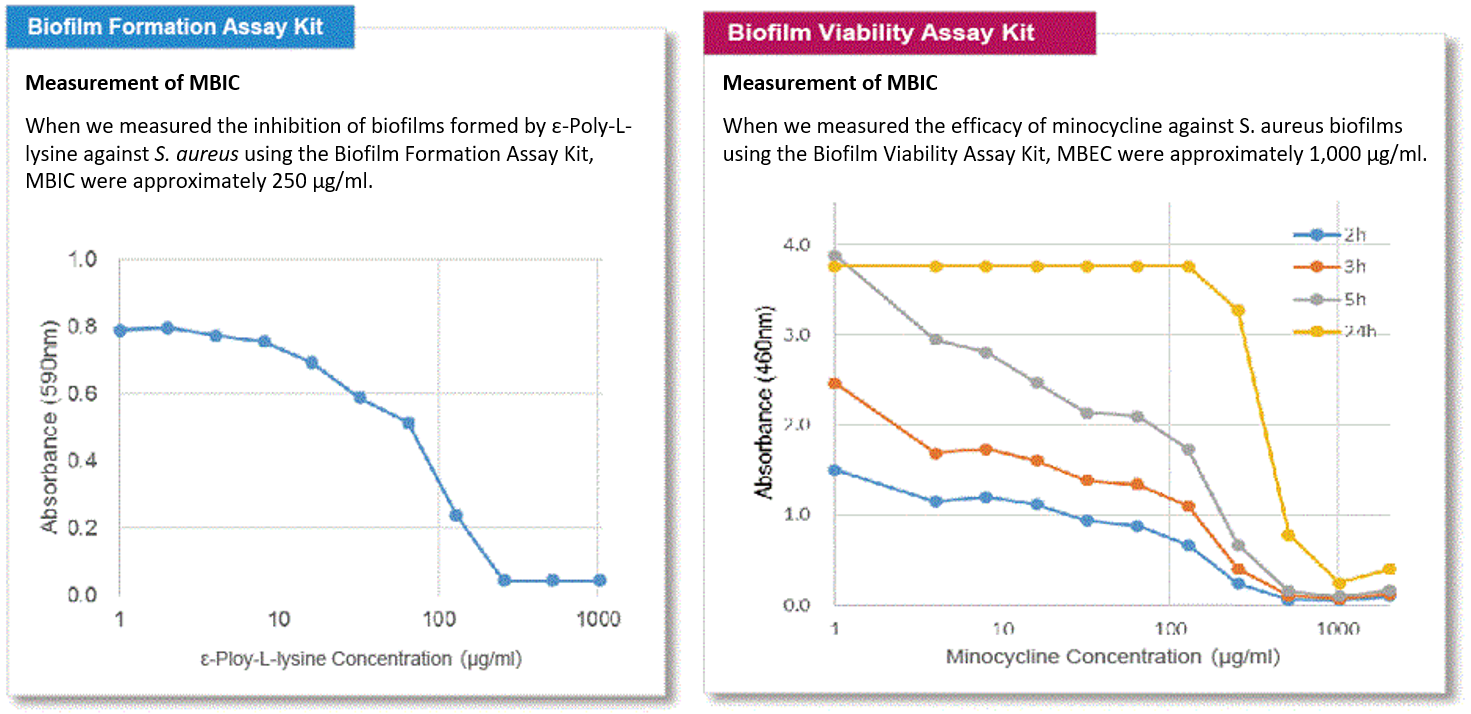
Indicators for drug-mediated inhibition of biofilm formation or antimicrobial effect of biofilms include MBIC (minimum biofilm inhibitory concentrations) and MBEC (minimum biofilm eradication concentrations). MBIC and MBEC of S. aureus were measured using each kit.
References
| No. | | Reference |
|
| 1) | Streptococcus mutans (ATCC 35668) |
I. Okamoto, H. Miyaji, S. Miyata, K. Shitomi, T. Sugaya, N. Ushijima, T. Akasaka, S. Enya, S. Saita and H. Kawasaki, "Antibacterial and Antibiofilm Photodynamic Activities of Lysozyme-Au Nanoclusters/Rose Bengal Conjugates", ACS Omega, 2021, doi:10.1021/acsomega.1c00838. |
| 2) | Acinetobacter baumannii |
Y. Sato, T. Ubagai, S. Tansho-Nagakawa, Y. Yoshino and Y.Ono, "Effects of colistin and tigecycline on multidrug-resistant Acinetobacter baumannii biofilms: advantages and disadvantages of their combination", Scientific Reports, 2021, 11, 11700. |
| 3) | Staphylococcus aureus |
S. Zhang, X. Qu, J. Jiao, H. Tang, M. Wang. Y. Wang, H. Yang, W. Yuan and B. Yue, "Felodipine enhances aminoglycosides efficacy against implant infections caused by methicillin-resistant Staphylococcus aureus, persisters and biofilms", Bioact. Mater. 2021 doi: 10.1016/j.bioactmat.2021.11.019. |
| 4) | Vibrio parahaemolyticus |
M. Billaud, F. Seneca, E. Tambutté and D. Czerucka, "An Increase of Seawater Temperature Upregulates the Expression of Vibrio parahaemolyticus Virulence Factors Implicated in Adhesion and Biofilm Formation", Frontiers in Microbiology, 2022, 13, 840628. |
Q & A
-
Q
How can I examine the conditions on biofilm formation?
-
A
A1. Test items for conditions on biofilm formation include types of culture media, seeding density of microorganisms, frequency of media changes, incubation time, incubation temperature, and others.
We show examples of optimizing the following test items: types of culture media, seeding density of microorganisms, frequency of media changes, and incubation time, as below.
If you optimize incubation temperature, it is necessary to use a number of the Biofilm Formation Assay Kits (Product code: B601).Measure the amount of biofilm formation – ①
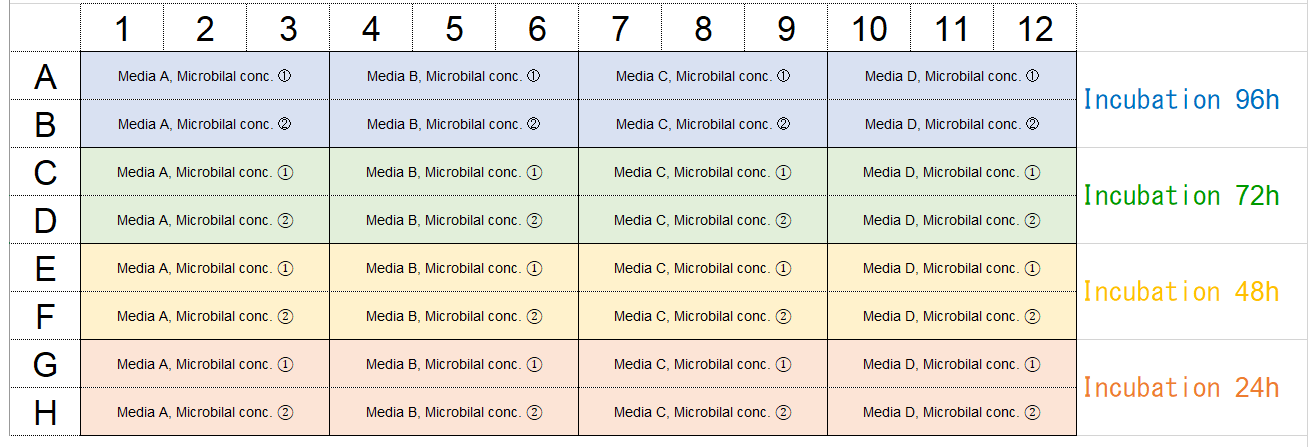
Under the following optimized conditions: types of culture media, seeding density of microorganisms, and media changes, the amount of biofilm formation can be confirmed by the experiments shown below.Example of plate arrangement
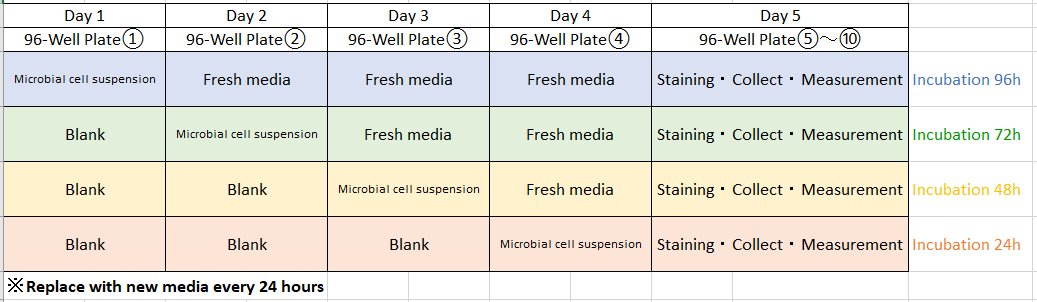
Experimental schedule
Procedure
(Step 1) Day 1: 96-well plate ①
In accordance with the reference layout shown above, fill each well (rows A and B) of the 96-well plate with 180μl of microbial cell suspension with concentrations ① and ② made in culture media A through D.
Leave the wells (rows C through H) blank. Place a 96-peg lid on the 96-well plate and incubate the plate at the optimum growth temperature for the microorganism for 24 hours.
※ Examples
Microbial concentrations ① and ②: Approximately 107 CFU/ml and 106 CFU/ml, respectively.
(Step 2) Day 2: 96-well ②
In accordance with the reference layout shown above, fill each well (rows A and B) of the 96-well plate with 180μl of culture media without microorganism.
Fill each well (rows C and D) of the same 96-well plate with 180μl of microbial cell suspension with concentrations ① and ② made in culture media A through D. Leave the wells (rows E through H) blank.
Place the 96-peg lid (Step1) on the 96-well plate ② and incubate the 96-well plate ② at the optimum growth temperature for the microorganism for 24 hours.
(Step 3) Day 3: 96-well plate ③
In accordance with the reference layout shown above, fill each well (rows A through D) of the 96-well plate with 180μl of culture media without microorganism.
Fill each well (rows E and F) of the same 96-well plate with 180 μl of microbial cell suspension with concentrations ① and ② made in culture media A through D. Leave the wells (rows G and H) blank.
Place the 96-peg lid (Step1) on the 96-well plate ③ and incubate the 96-well plate at the optimum growth temperature for the microorganism for 24 hours.
(Step 4) Day 4: 96-well plate ④
In accordance with the reference layout shown above, fill each well (rows A through F) of the 96-well plate with 180μl of culture media without microorganism.
Fill each well (rows G and H) of the same 96-well plate with 180μl of microbial cell suspension with concentrations ① and ② made in culture media A through D.
Place the 96-peg lid (Step1) on the 96-well plate ④ and incubate the 96-well plate ④ at the optimum growth temperature for the microorganism for 24 hours.
(Step5) Perform experiments using a new 96-well plate, in accordance with Steps 2 through 6 which describe the measurements of biofilm formation/inhibition of biofilm formation in the technical manual.
Measure the amount of biofilm formation – ②
Under the following optimized conditions: types of culture media, seeding density of microorganisms, and media changes, the amount of biofilm formation can be confirmed by the experiments shown below.Example of plate arrangement

Experimental schedule
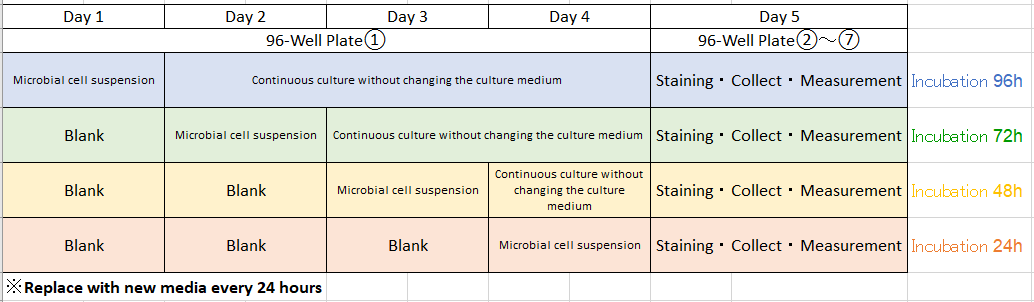
Procedure
(Step 1) Day 1: 96-well plate○1
In accordance with the reference layout shown above, fill each well (rows A and B) of the 96-well plate with 180μl of microbial cell suspension with concentrations ① and ② made in culture media A through D.Leave the wells (rows C through H) blank.
Place a 96-peg lid on the 96-well plate and incubate the plate at the optimum growth temperature for the microorganism for 24 hours.
※ Examples
Microbial concentrations ① and ②: Approximately 107 CFU/ml and 106 CFU/ml, respectively.
(Step2) Day 2: Remove the 96-peg lid. Allow cells to grow in the rows A and B of the 96-well plate○1 without changing the culture medium.
Fill each well (rows C and D) of the same 96-well plate with 180μl of microbial cell suspension with concentrations ① and ② made in culture media A through D. Leave the wells (rows E through H) blank.
Place the 96-peg lid back to the 96-well plate ①. Continuously incubate the 96-well plate ① at the optimum growth temperature for the microorganism for 24 hours.
(Step3) Day 3: Remove the 96-peg lid. Allow cells to grow in the rows (A through D) of the 96-well plate ①without changing the culture medium.
Fill each well (rows E and F) of the same 96-well plate with 180μl of microbial cell suspension with concentrations ① and ② made in culture media A through D. Leave the wells (rows G and H) blank. Place the 96-peg lid back to the 96-well plate ①. Continuously incubate the 96-well plate ① at the optimum growth temperature for the microorganism for 24 hours.
(Step 4) Day 4: Remove the 96-peg lid. Allow cells to grow in the rows (A through F) of the 96-well plate ① without changing the culture medium.
Fill each well (rows G and H) of the same 96-well plate with 180 μl of microbial cell suspension with concentrations ① and ② made in culture media A through D. Leave the wells (rows G and H) blank. Place the 96-peg lid back to the 96-well plate ①. Continuously incubate the 96-well plate ① at the optimum growth temperature for the microorganism for 24 hours.
(Step 5): Perform experiments using a new 96-well plate, in accordance with Steps 2 through 6 which describe the measurements of biofilm formation/inhibition of biofilm formation in the technical manual.
-
Q
What types of microbial species have been tested?
-
A
A2. Dojindo Laboratories has evaluated the following microbial species that formed biofilms using the Biofilm Formation Assay Kit (this product) and the Biofilm Viability Assay Kit (Product code: B603).
– Staphylococcus aureus
– Pseudomonas aeruginosa
– Escherichia coli
– Streptococcus mutans
– Porphyromonas gingivalisPlease refer to the technical manual, in terms of culture conditions for the above microbial species, experimental examples, etc.
-
Q
When I evaluate inhibition of biofilm formation and anti-biofilm drug efficacy, what is the approximate amount of biofilm formation required for experiment?
-
A
A3. In the Biofilm Formation Assay Kit (Product code: B601), please consider the amount of biofilm formation as a rough indication when a value of absorbance (590 nm) for crystal violet solution obtained after the final step is greater than 0.5.
Handling and storage condition
| Ambient temperature |