Annexin V Apoptosis Plate Assay Kit
Apoptosis (Annexin V) Plate Assay Kit
- Easy identification of cell death pathways in combination with other Dojindo’s kits
- Multiple indicators can be measured in combination on the same plate
- Accurate plate assay without the need for washing.
-
Product codeAD12 Annexin V Apoptosis Plate Assay Kit
| Unit size | Price | Item Code |
|---|---|---|
| 100 tests | $280.00 | AD12-10 |
| 100 tests | Annexin V - FITC Quenching Buffer |
×1 11 ml×1 |
|---|
Analysis of cell death in detail
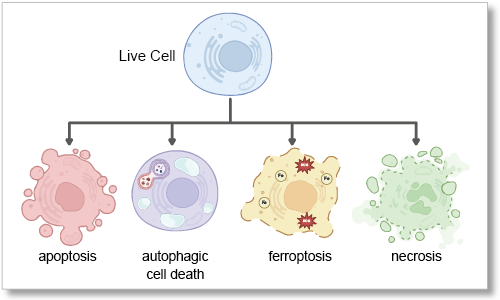
Cell death has been the subject of research for many years because of its role in the pathogenesis of several diseases. Traditionally, cell death has been divided into two types, apoptosis and necrosis, depending on the situation and the stimulus that triggers its induction. In recent years, however, cell death that follows a different pathway from apoptosis and necrosis, such as ferroptosis and autophagic cell death, has been discovered. Detailed analysis of cell death pathway is being studied as an approach to developing treatments for disease.
Description
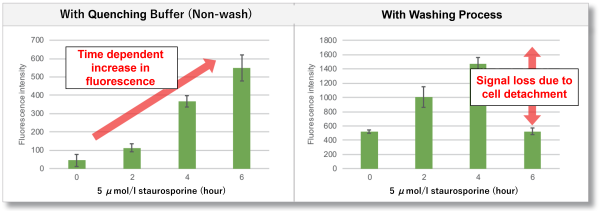
In normal cells, during the early stages of apoptosis, the membrane structure of phosphatidylserine (PS) present in the cells changes and it becomes exposed outside the cells. Previous measurements of PS using a plate reader have tended to detach cells as cell death is induced, which can lead to problems such as variation and reduction in fluorescence intensity due to washing procedures. This kit contains FITC-labelled Annexin V, which binds to PS, and a quenching buffer, eliminating the need for washing. Therefore, this new washless PS detection method allows for easy and accurate apoptosis detection.
In addition, cell samples are separated into cells and culture media and measured with different indicators (using a combination of our kits), allowing more detailed analysis of cell death.
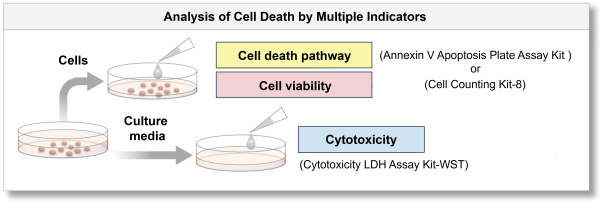
Cell Death Related Products
| Type | Product Name | Target | Detection Properties |
|---|---|---|---|
| Cell viability | Cell Counting Kit-8 | Dehydrogenase Activity |
Plate reader Colorimetric, λ= 450nm |
| Necrosis (Cytotoxicity) |
Cytotoxicity LDH Assay Kit-WST | Leaked LDH | Plate reader Colorimetric, λ= 490nm |
| Apoptosis | Annexin V Apoptosis Plate Assay Kit | Phosphatidylserine | Plate reader Ex: 488 nm / Em: 525 nm |
| Ferroptosis | FerroOrange | Intracellular Fe2+ | Microscopy, FCM, Plate reader Ex: 543 nm / Em: 580 nm |
| Ferroptosis | Mito-FerroGreen |
Mitochondrial Fe2+ |
Microscopy Ex: 505 nm / Em: 535 nm |
| Ferroptosis | Liperfluo | Lipid Peroxide | Microscopy, FCM Ex: 488 nm / Em: 500-550 nm |
Manual
Technical info
Apoptosis is a type of programmed cell death that plays an important role in maintaining homeostasis and developmental processes in plants and animals. For example, abnormal cells produced during cell generation are removed by apoptosis. It is known that in the early stages of apoptosis, PS present on the inner side of the cell membrane is transferred to the outer side of the cell membrane. This unique change makes it possible to identify apoptotic cells.
Annexin V is a protein that binds specifically to phosphatidylserine in the presence of calcium ions. By exploiting this property, apoptotic cells can be detected using fluorescently labelled Annexin V. Typically, apoptotic cells are detected using flow cytometry or fluorescence microscopy, but these are time-consuming to process multiple samples.
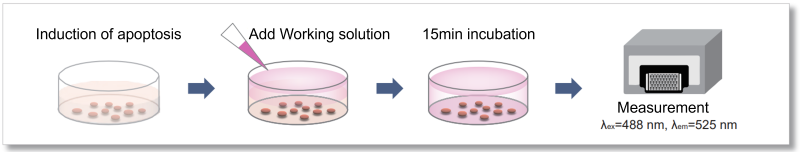
This kit contains fluorescently labelled Annexin V and a reagent (Quenching Buffer) that quenches the fluorescence of Annexin V not bound to PS, allowing rapid detection of multiple samples using a plate reader without the need for washing procedures.
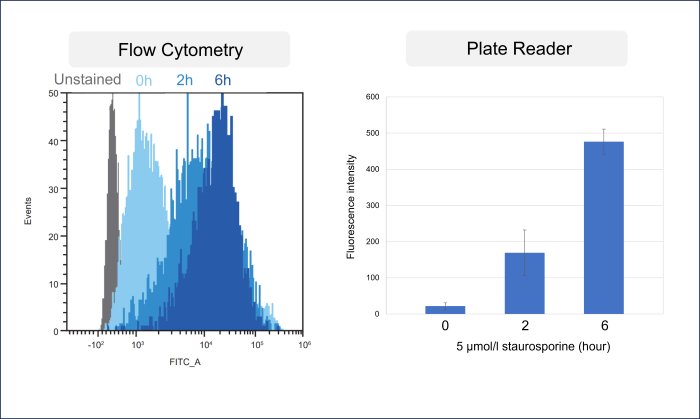
Comparison with flow cytometry
HeLa cells were treated with Staurosporine to induce apoptosis. One sample was stained with commercially available FITC-labeled Annexin V and then detected by a flow cytometer, while the other sample was detected by a plate reader using the Annexin V Apoptosis Plate Assay Kit. As a result, an increase in fluorescence intensity was confirmed with the progression of apoptosis in both the flow cytometer and the plate reader.
-
[Experimental conditions]
Cell type: HeLa cells
Staurosporine concentration: 5 µmol/l
Time: 0 to 6 hours[Detection conditions]
Plate reader: TECAN Infinite M200 PRO, bottom reading
Flow cytometer: SONY SA3800
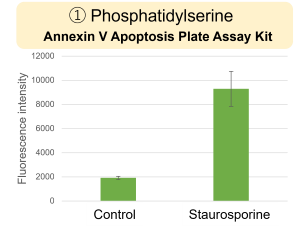
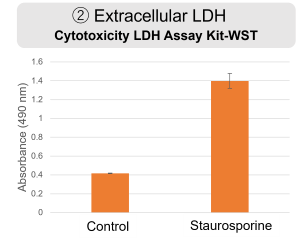
Experimental example: Changes in various indicators due to Staurosporine
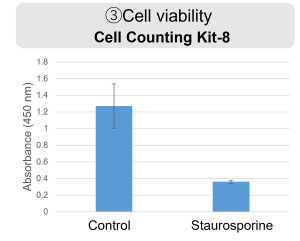
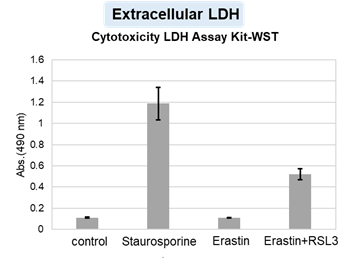
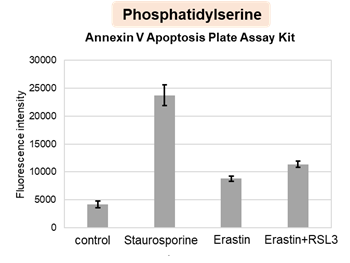
HepG2 cells were treated with staurosporine to induce apoptosis, and phosphatidylserine, extracellular LDH and cell proliferation were detected. Phosphatidylserine was measured as an apoptosis marker using the Annexin V Apoptosis Plate Assay Kit, extracellular LDH was measured as an indicator of dead cells using the Cytotoxicity LDH Assay Kit-WST , and cell proliferation was measured using the Cell Counting Kit-8. The results showed that staurosporine treatment increased phosphatidylserine and extracellular LDH, and decreased cell proliferation.
[Experimental Procedure]
1. HepG2 cells (2 x 104 cells /well) were seeded into a 96-well Black Plate (clear bottom) and cultured overnight in an incubator (37°C, 5% CO2) .
2. The medium was removed, and 200 μl of MEM medium (control) or 5 μmol/l staurosporine prepared in MEM medium was added, followed by incubation in an incubator (37°C, 5% CO2) for 24 hours .
3. 100 µl of the supernatant from the wells used for measuring phosphatidylserine was transferred to a 96-well clear plate, and ② extracellular LDH was measured according to the instructions for use with the Cytotoxicity LDH Assay Kit (non-homogeneous assay).
4. 60 μl of working solution was added to well ① for phosphatidylserine measurement on a 96-well Black Plate and incubate at room temperature for 15 minutes in the dark. The fluorescence intensity (Ex/Em = 488/525 nm) was then measured using a plate reader (bottom reading). The blank values were subtracted from the obtained values to calculate the fluorescence intensity derived from Annexin V - FITC.
5. After removing 100 μl of the supernatant from the well for measuring cell proliferation (③) of the 96-well Black Plate, measurements were performed according to the instructions for the Cell Counting Kit-8 (color reaction for 2 hours followed by measurement of absorbance).
[Detection conditions]
Plate reader: TECAN Infinite M200 PRO, bottom reading
Experimental example: Changes in various indicators of cell death induced by drugs
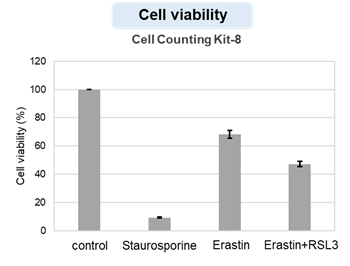
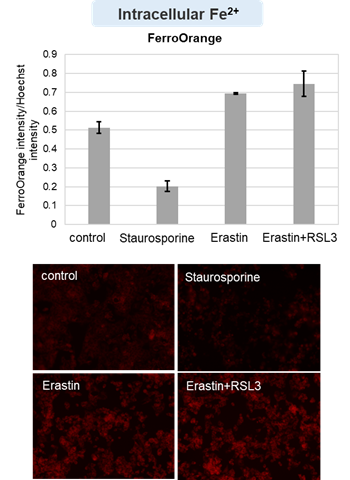
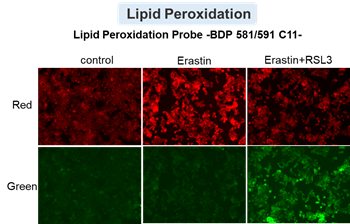
HepG2 cells treated with the apoptosis-inducing agent staurosporine or the ferroptosis-inducing agents Erastin and RSL3. After treatment, extracellular LDH, phosphatidylserine, cell viability, intracellular Fe2+ and lipid peroxidation were determined.
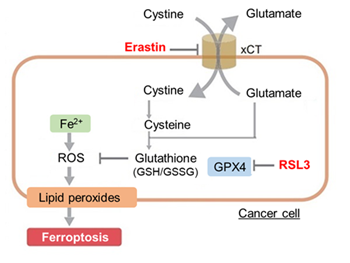
The results showed that apoptosis-induced cells treated with staurosporine showed an increase in phosphatidylserine, a decrease in cell viability and an increase in extracellular LDH, indicating that cell death had occurred. On the other hand, intracellular Fe2+, an indicator of ferroptosis, remained unchanged. In cells treated with Erastin, a ferroptosis inducer, intracellular Fe2+ increased and cell viability decreased, but extracellular LDH and lipid peroxidation (lipid peroxidation: decrease in red fluorescence and increase in green fluorescence) did not increase. In cells in which ferroptosis was more strongly induced by co-treatment with RSL3 in addition to Erastin, increased intracellular Fe2+ and lipid peroxidation were observed. Moreover, decreased cell viability and increased dead cells were detected. Meanwhile, phosphatidylserine showed a lower rate of increase during ferroptosis induction compared to apoptosis-induced cells. These results suggest that cell death can be distinguished by evaluating a combination of cell death indicators.
[Products in use]
Extracellular LDH : Cytotoxicity LDH Assay Kit-WST (Product code: CK12)
Phosphatidylserine: Annexin V Apoptosis Plate Assay Kit(Product code: AD12)
Cell viability : Cell Counting Kit-8 (Product code: CK04)
Intracellular Fe2+ : FerroOrange (Product cose: F374) *Normalized with Hoechst 33342 fluorescence intensity
Lipid peroxidation : Lipid Peroxidation Probe -BDP 581/591 C11- (Product code: L267)
[Experimental conditions]
Cell type: HepG2 cell(2×104 cells/well)
Drugs: Staurosporin(5 μmol/l), Erastin(25 µmol/l), Erastin+RSL3(both 25 µmol/l) *Diluted in serum-free medium
Q & A
-
Q
How many samples can be measured by one kit?
-
A
If each sample is measured in triplicate, a maximum of 31 samples can be measured.
However, if there are samples in different media, a blank measurement is required for each medium.

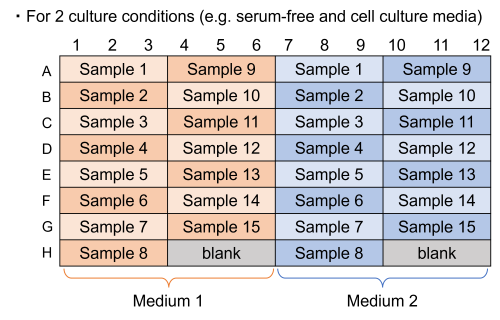
-
Q
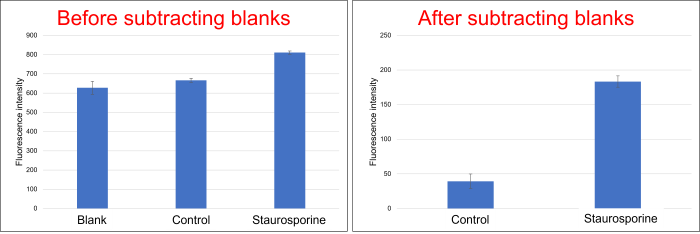
Is this a problem as the blank seems to have a high background?
-
A
The blank may have a similar fluorescence intensity to the control, which does not induce apoptosis. This is not a problem with the reagent, so please subtract the blank fluorescence intensity from the results as described in the instructions for use.
-
Q
The difference in fluorescence intensity between control and tested samples is small. What should I do?
-
A
Please check the following two points.
1. Cell numbers
It has been confirmed that fluorescence intensity varies depending on the number of cells. We recommend using 1-5 x 104 cells/well as a guideline for cell number.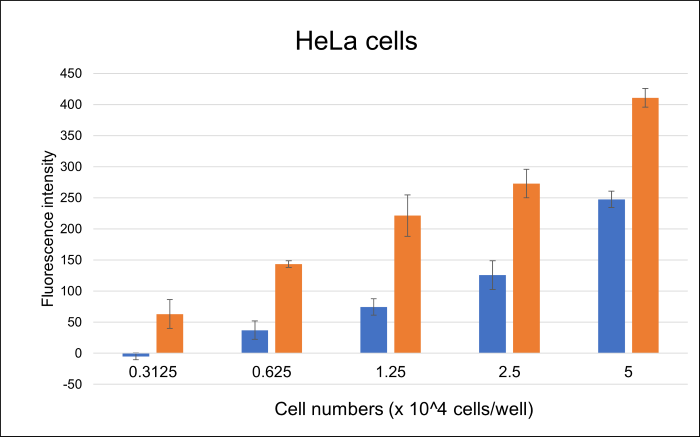
Cell numbers (×104 cells/well) 0.3125 0.625 1.25 2.5 5.0 S/N ー 3.9 3.0 2.2 1.7 2. Treatment time
The stimulation time required for phosphatidylserine to become positive varies depending on the cell type.
Please consider the optimal stimulant treatment time.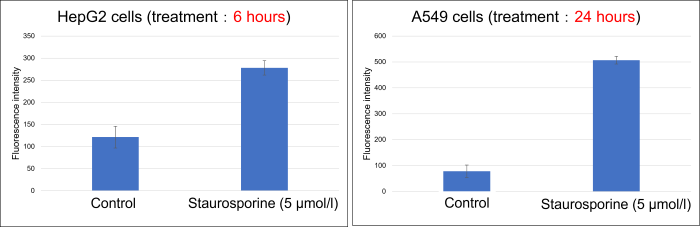
Handling and storage condition
| Terms and Conditions Storage conditions: Refrigerate, Handling conditions: Nitrogen replacement, Be careful of moisture absorption, Toxic substances: Non-medicinal poisons |