Previous Science Note
| Mitochondria are increasingly recognized as critical regulators of glial cell function in the central nervous system, influencing both neuroinflammatory responses and neuronal integrity. This Science Note reviews recent advances in our understanding of how mitochondrial activity within glial cells shapes their pathological and protective roles in the inflamed brain and provides insights into potential therapeutic strategies targeting glial mitochondrial metabolism. | ||||||||||||||||||||||||||
|
Mitochondrial complex I activity in microglia sustains neuroinflammation (Nature, 2025) Highlighted technique: To elucidate the relationship between complex I activity and reverse electron transport (RET)-mediated ROS production in microglia, this study used integrated transcriptomic, proteomic and metabolomic analyses to comprehensively assess changes in electron transport chain enzyme expression, metabolic flux and antioxidant responses. Related technique Mitochondrial Superoxide Detection, Mitochondrial Membrane Potential Detection |
||||||||||||||||||||||||||
|
Loss of fatty acid degradation by astrocytic mitochondria triggers neuroinflammation and neurodegeneration (Nature Metabolism, 2023) Highlighted technique: In this study, astrocyte-specific Tfam knockout mice were generated to model selective mitochondrial OXPHOS deficiency in astrocytes. Using this model, the authors showed that loss of astrocytic mitochondrial OXPHOS leads to cognitive impairment and neuroinflammation. Related technique Lipid Droplet Detection, Glycolysis/Oxidative Phosphorylation Assay |
||||||||||||||||||||||||||
|
Microglia rescue neurons from aggregate-induced neuronal dysfunction and death through tunneling nanotubes (Neuron, 2024) Highlighted technique: In this study, neurons and microglia were co-cultured to observe the intercellular transfer of materials such as mitochondria, alpha-synuclein and tau via tunneling nanotubes (TNTs). These transfers were captured using fluorescent labelling and live cell imaging, allowing real-time observation of TNT-mediated dynamics. Related technique Oxygen Consumption Rate Assay, Total ROS Detection |
||||||||||||||||||||||||||
Related Techniques (click to open/close)
|
||||||||||||||||||||||||||
Application Note I (click to open/close)
|
||||||||||||||||||||||||||
|
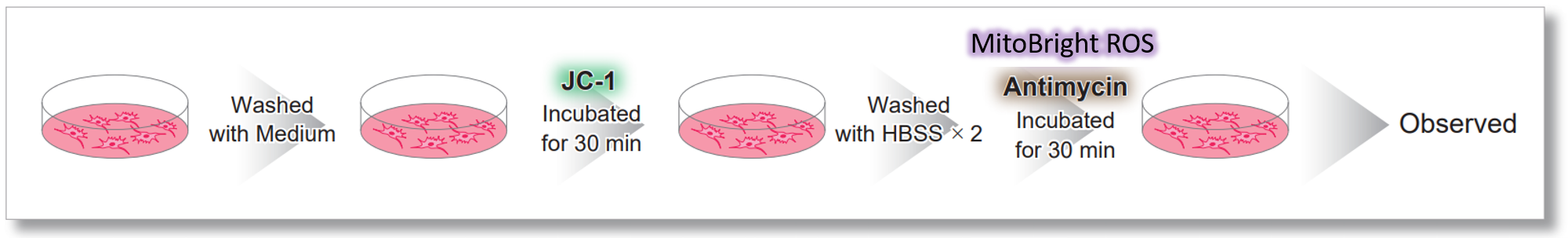
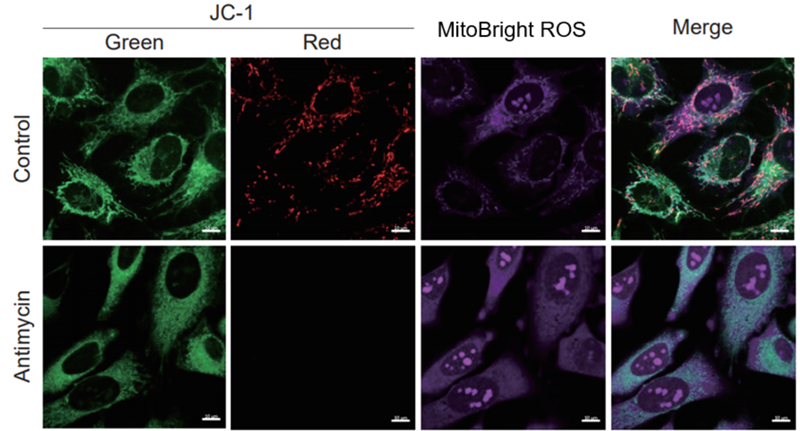
After HeLa cells were washed with HBSS, co-stained with MitoBright ROS Deep Red and mitochondrial membrane potential staining dye (JC-1: code MT09), and the generated mitochondrial ROS and membrane potential were observed simultaneously. As a result, the decrease in mitochondrial membrane potential and the generation of mitochondrial ROS are simultaneously observed. |
|
|
|
|
Application Note II (click to open/close)
> Inhibition of Mitochondrial Electron Transport Chain
|
|
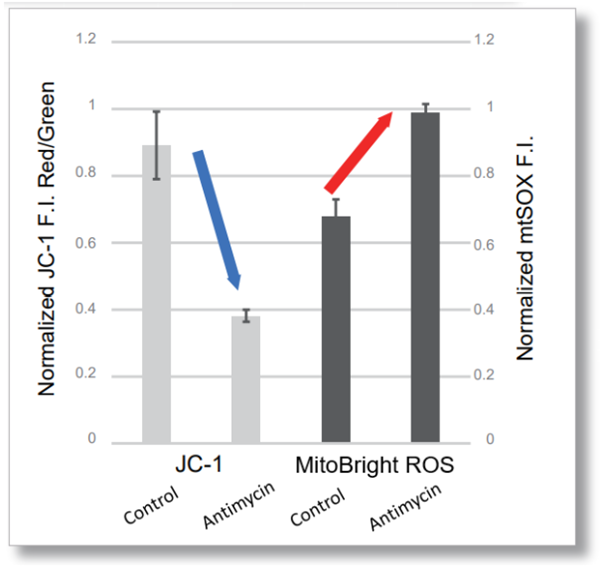
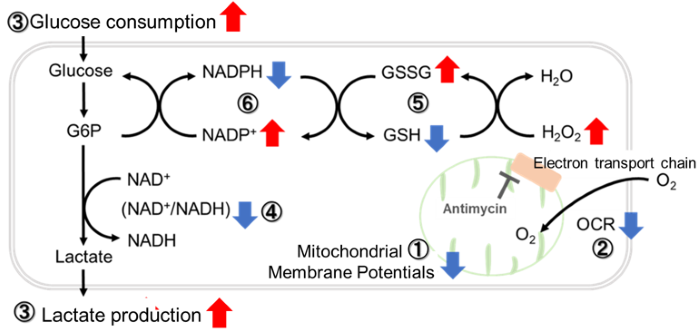
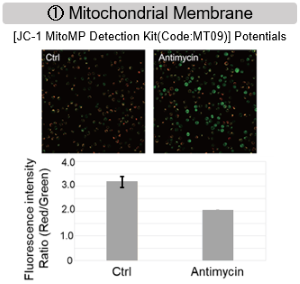
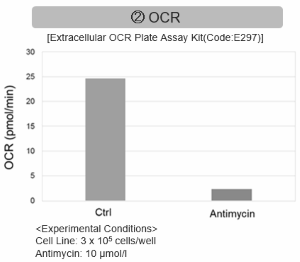
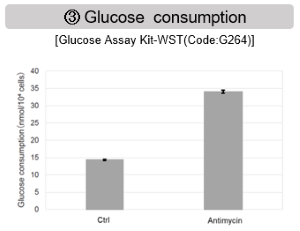
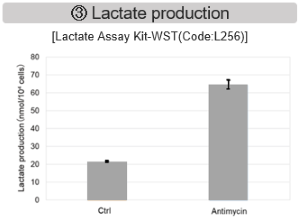
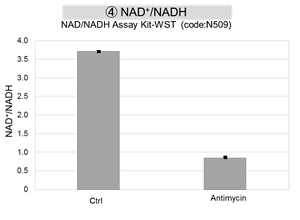
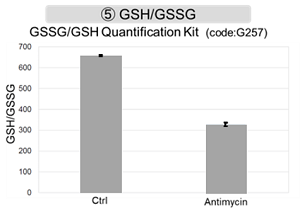
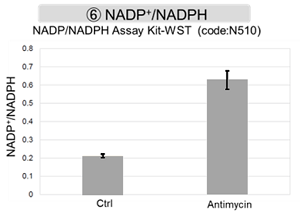
Antimycin stimulation of Jurkat cells was used to evaluate the changes in cellular state upon inhibition of the mitochondrial electron transport chain using a variety of indicators. The results showed that inhibition of the electron transport chain resulted in (1) a decrease in mitochondrial membrane potential and (2) a decrease in OCR. In addition, (3) the NAD+/NADH ratio of the entire glycolytic pathway decreased due to increased metabolism of pyruvate to lactate to maintain the glycolytic pathway, (4) GSH depletion due to increased reactive oxygen species (ROS), and (6) increase in the NADP+/NADPH ratio due to decreased NADH required for glutathione biosynthesis were observed.
|
||




 <Imaging Conditions>(Confocal microscopy)
<Imaging Conditions>(Confocal microscopy)