EMCS
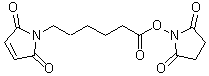
Cross-Linker
-
Product codeE018 EMCS
-
CAS No.55750-63-5
-
Chemical nameN-(6-MaleimidOcaproyloxy)succinimide
-
MWC14H16N2O6=308.29
| Unit size | Price | Item Code |
|---|---|---|
| 50 mg | Find your distributors | E018-10 |
| 100 mg | Find your distributors | E018-12 |
Description
Cross-linking Reaction

Product Description
Hetero-bifunctional cross-linking reagents have activated esters and maleimide reactive groups. These functional groups react with amines and sulfhydryl groups of proteins, respectively. Enzyme-labeled haptens are prepared using hetero-bifunctional cross-linking reagents such as EMCS or GMBS. The cross-linking reaction requires neutral pH and mild temperature because it is necessary to maintain enzyme activity and antibody titers in the cross-linking reaction. Hetero-bifunctional cross-linking reagents with 3, 5, 7, or 10 linear carbon chains are available. These linear aliphatic chains act as spacers between the two reactive sites and their water-soluble reagents. They are more stable than the aromatic cross-linking reagents such as succinimidyl-4-N-maleimidobenzoate in a wider pH range.
Conjugation of Macromolecules with Hetero-Bifunctional Cross-Linking Reagent

Technical info
References
1. S. Yoshitake, Y. Yamada, E. Ishikawa and R. Masseyeff, Conjugation of Glucose Oxidase from Aspergillus Niger and Rabbit Antibodies Using N-Hydroxysuccinimide Ester of N-(4-Carboxycyclohexylmethyl)-Maleimide, Eur. J. Biochem., 1979, 101, 395.
2. T. Kitagawa, T. Shimozono, T. Aikawa, T. Yoshida and H. Nishimura, Preparation and Characterization of Hetero-bifunctional Cross-linking Reagents for Protein Modifications, Chem. Pharm. Bull., 1981, 29, 1130.
3. S. Yoshitake, M. Imagawa, E. Ishikawa, Y. Niitsu, I. Urushizaki, M. Nishimura, R. Kanazawa, H. Kurosaki, S. Tachibana, N. Nakazawa and H. Ogawa, Mild and Efficient Conjugation of Rabbit Fab Eand Horseradish Peroxidase Using a Maleimide Compound and Its Use for Enzyme Immunoassay, J. Biochem., 1982, 92, 1413.
4. E. Ishikawa, M. Imagawa, S. Hashida, S. Yoshitake, Y. Hamaguchi and T. Ueno, Enzyme-Labelling of Antibodies and Their Fragments for Enzyme Immunoassay and Immunohistochemical Staining, J. Immunol., 1983, 4, 209.
5. I. Weeks, A. K. Campbell and J. S. Woodhead, Two-site Immunochemiluminometric Assay for Human α1-Fetoprotein, Clin. Chem., 1983, 29, 1480.
6. S. Hashida, M. Imagawa, S. Inoue, K. Ruan and E. Ishikawa, More Useful Maleimide Compounds for the Conjugation of Fab Eto Horseradish Peroxidase through Thiol Groups in the Hinge, J. Appl. Biochem., 1984, 6, 56.
7. S. Inoue, M. Imagawa, S. Hashida, K. Ruan and E. Ishikawa, A Small Scale Preparation of Affinity-Purified Rabbit Fab EHorseradish Peroxidase Conjugate for Enzyme Immunoassay, Anal. lett., 1984, 17, 229.
8. M. Koizumi, K. Endo, M. Kunimatsu, H. Sakahara, T. Nakashima, Y. Kawamura, Y. Watanabe, T. Saga, J. Konishi, T. Yamamura, S. Hosoi, S. Toyama, Y. Arano and A. Yokoyama, Ga-Labeled Antibodies for Immunoscintigraphy and Evalution of Tumor Targeting of Drug-Antibody Conjugatesin Mice, Cancer Res., 1988, 48, 1189.
9. T. Kohno and E. Ishikawa, Novel Enzyme Immunoassay(Immune Complex Transfer Enzyme Immunoassay) for Anti-insulin IgG in Guinea Pig Serum, Anal. lett., 1988, 21, 1019.
Handling and storage condition
| Appearance: | White to slightly yellowish white powder |
|---|---|
| Purity (HPLC): | ≧ 90.0 % |
| Solubility in Dimethylformamide: | To pass test (clear, colorless) |
| 0-5°C |








