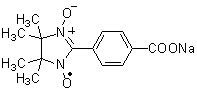
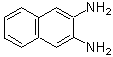
2,3-Diaminonaphthalene (for NO detection)
NO Detection
-
Product codeD418 2,3-Diaminonaphthalene (for NO detection)
-
CAS No.771-97-1
-
Chemical name2,3-Diaminonaphthalene
-
MWC10H10N2=158.2
| Unit size | Price | Item Code |
|---|---|---|
| 10 mg | $81.00 | D418-10 |
Product Description
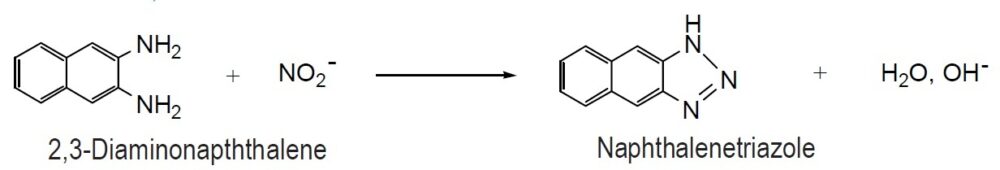
The Griess assay is a simple and popular method for detecting NO concentration. 2,3-Diaminonaphthalene (DAN) is a highly sensitive alternative to the Griess assay. The DAN method is 50-100 times more sensitive than the Griess assay: While the detection limit of the Griess assay is 1 mM, the limit of the DAN method is 10-50 nM. DAN reacts with NO2– in acidic conditions to produce fluorescent naphthalenetriazole. The wavelength of the emission maximum of naphthalenetriazole is 410 nm. However, detection at 450 nm is recommended to avoid fluorescent blanks and increase sensitivity. The fluorescent background of DAN is low for maximum sensitivity. The optimal reaction conditions of DAN with NO2– have been determined. The reaction should proceed at pH 2 at room temperature for 5 minutes, and the resulting fluorescence of naphthalenetriazole should be determined at a pH of 10 or more. DAN is a photosensitive reagent and sometimes becomes dark brown colored crystals. Since this brown product cannot be utilized for the fluorescent detection, recrystallization is necessary.
Reaction of 2,3-Diaminonaphthalene with NO2
Technical info
1. Dissolve 50 μg DAN in 1 ml 0.62 M HCl to prepare 0.31 mM DAN solution.a)
2. Mix 10 μl DAN solution with 100 μl NaNO2 solution (0-10 mM) or sample solution. Incubate the mixture at room temperature for 10-15 minutes.
3. Add 5 μl 2.8 M NaOH solution to the reaction solution.b)
4. Dilute 100 μl of this solution with 4 ml water, followed by fluorescent measurement with excitation wavelength at 365 nm and emission wavelength at 450 nm.
5. Prepare a calibration curve using this data where the X-axis is NaNO2 concentration and the Y-axis is fluorescence intensity. Then, use this calibration curve to determine the NO2 concentration of the sample solution.
a) Acidic conditions are required for a rapid reaction.
b) Basic conditions (pH 10 or higher) are required for a high fluorescence signal.
References
C. R. Sawicki, Anal. Lett., 4, 761 (1971);
P. Damiani, et al., Talanta, 8, 649 (1986);
W. R. Tracey, et al., J. Pharmacol. Exp. Ther., 252, 922 (1990);
J. S. Pollock, et al., Proc. Natl. Acad. Sci. USA, 88, 10480 (1991);
T. P. Misko, et al., Anal. Biochem., 214, 11 (1993);
R. G. Tilton, et al., Invest. Ophthamol. Vis. Sci., 35, 3278 (1994);
T. A. Mayer, et al., J. Surg. Res., 58, 570 (1995);
Y. Kono, et al., Biochem. J., 312, 947 (1995);
R. Metheringham, et al., Microbiology, 143, 2647 (1997);
A. M. Rao, et al., Brain Res., 793, 265 (1998);
N. Nakatsubo, et al., Biol. Pharm. Bull., 21, 1247 (1998);
D. Jourd’heuil, et al., Arch. Biochem. Biophys., 365, 92 (1999).
Handling and storage condition
| Appearance: | White to pale yellowish brown powder |
|---|---|
| Solubility in Dilute hydrochloric acid: | To pass test (clear, colorless to slightly pink) |
| m.p.: | 185 - 200℃ |
| Suitability for NO quantification: | To pass test |
| IR spectrum: | Authentic |
| -20°C, Protect from light |