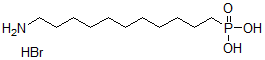11-AUPA
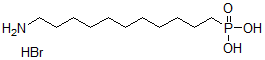
Self Assembled Monolayer Reagent
-
Product codeA517 11-AUPA
-
CAS No.45000
-
Chemical name340-09791
-
MWC11H27BrNO3P=332.21
| Unit size | Price | Item Code |
|---|---|---|
| 10 mg | $201.00 | A517-10 |
| 100 mg | $548.00 | A517-12 |
Structural Formula
Structural Formula
Product Description
Phosphonic acid derivatives are used for surface modification on oxidized metals such as Al2O31), TiO22), ZrO23), SiO24), Mica5), stainless(SS316L) 6), nitinol7), hydroxyapatite8), ZnO9), ITO10,11).
For a long time, organosilanes have been used to form self assembled monolayer (SAM) on the metal oxide. However, it is not always adaptable in the applications due to the poor stability and polymerization of the reagent with each other. On the other hand, phosphonic acid derivatives equally form a SAM on the metal oxide despite being very stable compounds. Also, phosphonic acid derivatives have been reported to use formation of more stable and dense SAM than organosilanes. Schwartz et. al. shows the phosphonate SAM perform stable and higher density by four times than that of the organosilane in alkaline solution when a SAM of 11-HUPA is formed on Titanium oxide film to modify the fluorescent molecules2). The phosphonic acid derivative mechanistically bind to a high density on titanium oxide film because a phosphonic acid derivative produce OH by a proton transfer to the substrate while an organosilne react only with OH group present in the titanium oxide film12).
Product Description
Phosphonic acid derivatives are used for surface modification on oxidized metals such as Al2O31), TiO22), ZrO23), SiO24), Mica5), stainless(SS316L) 6), nitinol7), hydroxyapatite8), ZnO9), ITO10,11).
For a long time, organosilanes have been used to form self assembled monolayer (SAM) on the metal oxide. However, it is not always adaptable in the applications due to the poor stability and polymerization of the reagent with each other. On the other hand, phosphonic acid derivatives equally form a SAM on the metal oxide despite being very stable compounds. Also, phosphonic acid derivatives have been reported to use formation of more stable and dense SAM than organosilanes. Schwartz et. al. shows the phosphonate SAM perform stable and higher density by four times than that of the organosilane in alkaline solution when a SAM of 11-HUPA is formed on Titanium oxide film to modify the fluorescent molecules2). The phosphonic acid derivative mechanistically bind to a high density on titanium oxide film because a phosphonic acid derivative produce OH by a proton transfer to the substrate while an organosilne react only with OH group present in the titanium oxide film12).
References
1) T. Hauffman, O. Blajiev, J. Snauwaert, C. van Haesendonck, A. Hubin, H. Terryn, "Study of the self-assembling of n-octylphosphonic acid layers on aluminum oxide", Langmuir, 2008, 24 (23), 13450.
2) P. Thissen, M. Valtiner, G. Grundmeier, "Stability of Phosphonic Acid Self-Assembled Monolayers on Amorphous and Single-Crystalline Aluminum Oxide Surfaces in Aqueous Solution", Langmuir, 2010, 26 (1), 156.
3) W. Gao, L. Reven, "Solid-state NMR-studies of self-assembled monolayers", Langmuir, 1995, 11 (6), 1860.
4) S. Marcinko, A. Y. Fadeev, "Hydrolytic Stability of Organic Monolayers Supported on TiO2 and ZrO2", Langmuir, 2004, 20 (6), 2270.
5) J. Schwartz, M. J. Avaltroni, M. P. Danahy, B. M. Silverman, E. L. Hanson, J. E. Schwarzbauer, K. S. Midwood, E. S. Gawalt, " Cell attachment and spreading on metal implant materials", J. Mat. Sci. Eng. C, 2003, 23, 395.
6) B. M. Silverman, K. A. Wieghaus, J. Schwartz, "Comparative properties of siloxane vs phosphonate monolayers on a key titanium alloy", Langmuir, 2005, 21 (1), 225.
7) N. Adden, L. J. Gamble, D. G. Castner, A. Hoffmann, G. Gross, H. Menzel, "Phosphonic Acid Monolayers for Binding of Bioactive Molecules to Titanium Surfaces", Langmuir, 2006, 22, 8197.
8) E. L. Hanson, J. Schwartz, B. Nickel, N. Koch, M. F. Danisman, "Bonding self-assembled, compact organophosphonate monolayers to the native oxide surface of silicon", J. Am. Chem. Soc. 2003, 125 (51), 16074.
9) M. Dubey, T. Weidner, L. J. Gamble, D. G. Castner, "Structure and Order of Phosphonic Acid-Based Self-Assembled Monolayers on Si(100)", Langmuir, 2010, 26 (18), 14747.
10) A. Vega, P. Thissen, Y. J. Chabal, "Environment-Controlled Tethering by Aggregation and Growth of Phosphonic Acid Monolayers on Silicon Oxide", Langmuir, 2012, 28, 8046.
11) P. Thissen, A. Vega, T. Peixoto, Y. J. Chabal, "Controlled, Low-Coverage Metal Oxide Activation of Silicon for Organic Functionalization: Unraveling the Phosphonate Bond", Langmuir, 2012, 28 (50), 17494.
12) J. T. Woodward, A. Ulman, D. K. Schwartz, "Self-assembled monolayer growth of octadecylphosphonic acid on mica", Langmuir, 1996, 12 (15), 3626.
13) A. Raman, M. Dubey, I. Gouzman and E. S. Gawalt, "Formation of self-assembled monolayers of alkylphosphonic acid on the netive oxide surface of SS316L", Langmuir, 2006, 22, 6469.
14) G. Zorn, R. Adadi, R. Brener, V. A. Yakovlev,| I. Gotman, E. Y. Gutmanas, C. N. Sukenik, "Tailoring the Surface of NiTi Alloy Using PIRAC Nitriding Followed by Anodization and Phosphonate Monolayer Deposition", Chem. Mater. 2008, 20, 5368.
15) R. Quinones and E. S. Gawalt, "Polystyrene formation on monolayer-modified nitinol effectively controls corrosion", Langmuir, 2008, 24, 10858.
16) S. C. D’Andrea and Al. Y. Fadeev, "Covalent surface modification of calcium hydroxyapatite using n-alkyl- and n-fluoroalkylphosphonic acids", Langmuir, 2003, 19, 7904.
17) B. Zhang, T. Kong, W. Xu, R. Su, Y. Gao and G. Cheng, "Surface functionalization of zinc oxide by carboxyalkylphosphonic acid self-assembled monolayers", Langmuir, 2010, 26(6), 4514.
18) A. Sharma, B. Kippelen, P. J. Hotchkiss and S. R. Marder, "Stabilization of the work function of indium tin oxide using organic surface modifiers in organic light-emitting diodes", Appl. Phys. Lett., 2008, 93, 163308.
Handling and storage condition
| Appearance: | White to slightly yellow powder |
|---|---|
| Solubility in Methyl alcohol: | To pass test (clear, colorless) |
| NMR spectrum: | Authentic |
| Ambient temperature |