ALC
Metal Indicator
-
Product codeA006 ALC
-
CAS No.3952-78-1
-
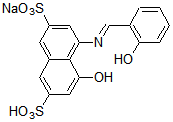
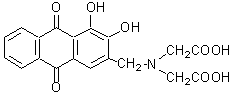
Chemical name3-[N,N-Bis(carboxymethyl)aminomethyl]-1,2-dihydroxyanthraquinone
-
MWC19H15NO8=385.32
| Unit size | Price | Item Code |
|---|---|---|
| 100 mg | $39.00 | A006-10 |
| 1 g | $157.00 | A006-12 |
Description
Product Description
ALC is utilized for colorimetric detection of fluoride ion. A red solution of the La(III) or Ce(III) complex turns blue in the presence of fluoride ions. The maximum wavelength of the fluoride complex is 620 nm at pH 4-5. As little as 0.1 to 1 ppm of fluoride ion can be determined using this method. The structure of this complex has been reported as La2L2F2, (La5L4F2)n or Ce5L4F4 (L: ALC). ALC is insoluble in alcohol and ether, slightly soluble in water, and easily soluble in alkaline water. The fluoride complex can be extracted with iso-amylalcohol. Since ALC is less toxic than Alizarin red, it is used to mark and trace young fish. The aqueous solution of ALC is yellow at pH < 6, red at pH 6 – 10, and blue violet at pH > 11. ALC can also be used to detect aluminum ions. pKa1(COOH )=2.40, pKa2(OH)=5.54, pKa3(NH+)=10.07, pKa4(OH)=11.98 (m=0.1).
References
1. R. Belcher, et al., A Study of the Cerium-Alizarin Complexan Fluoride Reaction. Talanta. 1961;8:853-862.
2. F. Ingman, Annotation the Acid Stability Constants of Alizarin Fluorine Blue. Talanta. 1973;20:135-138.
3. F. Ingman, Photometric Determination of Aluminium with Alizarin Fluorine Blue. Talanta. 1973;20:999-1007.
4. Y. Yamashita, et al., Effects of Release Size on Survival and Growth of Japanese Flounder Paralichthys Olivaceus in Coastal Waters of Iwate Prefecture, Northeastern Japan. Mar Ecol Prog Ser. 1994;105:269-276.
Handling and storage condition
| Appearance: | Yellowish orange to yellowish brown powder |
|---|---|
| Solubility in NaOH solution: | To pass test (clear, deep reddish purple) |
| Absorbance: | ≧ 0.420 (around 430 nm) |
| Sulfated ash: | ≦ 0.50 % |
| IR spectrum: | Authentic |
| Ambient temperature |