General Information
The cystine transporter xCT is a crucial transporter that maintains cellular redox balance by regulating glutathione synthesis via cystine uptake. xCT is highly expressed in several types of cancer cells, and is expected to be a therapeutic target for cancer. For a long time, the conventional method used to assess xCT activity has been a cystine uptake assay using radioisotopes. However, this method requires special handling facilities and disposal of radioactive materials.
The Cystine Uptake Assay Kit allows a simple fluorescence-based assay for cystine uptake via the xCT. The Cystine Analog (CA) in this kit can be taken up into cells via xCT, and the incorporated CA can be specifically detected using the Fluorescent Probe and Reducing Agent. Thus, the xCT activity can be measured easily.
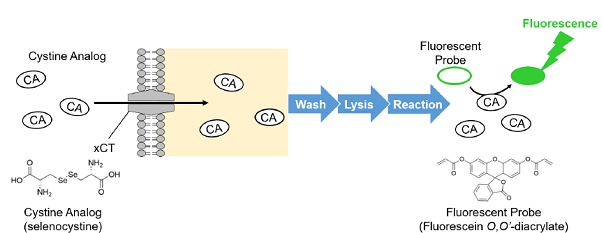
Figure 1. Principle of Cystine Uptake Assay Kit
(Patent pending)
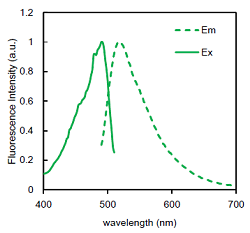
Figure 2. Excitation and emission spectra of the Fluorescent Probe
Kit Contents
| 20 tests | 100 tests | |
| Cystine Analog Solution | 45 μl x 1 | 225 μl x 1 |
| Fluorescent Probe | x 1 | x 1 |
| Reducing Agent | x 1 | x 2 |
| Assay Buffer | 5 ml x 1 | 25 ml x 1 |
Storage Condition
Store at -20°C
Required Equipment and Materials
-
- Fluorescence microplate reader (Ex/Em = 490/535 nm)
- 96-Well black microplate
- Plate seals
- Incubator (37°C)
- 20-200 and 100-1000 μl micropipettes
-
- 20-200 μl multichannel pipette
- Medium (cystine-free, serum-free)
- Phosphate-buffered saline (PBS)
- Methanol
- Dimethylsulfoxide (DMSO)
Precaution
- ・Please tap the tube before opening, and open it with care. The content may have relocated from the bottom of the tube during shipping.
- ・Multiple measurements (e.g., in triplicate) are recommended to obtain accurate data.
- ・When correcting the measured value (fluorescence intensity) by the number of cells or protein concentration, the methods of correction, and the appropriate sample solution, vary depending on whether they are adherent or floating cells. For details, please refer to the FAQ: “How can I correct the measured values (fluorescence intensity) for the number of cells or protein concentration?”
Preparation of Solutions
1. Preparation of CA uptake solution
Dilute Cystine Analog Solution 100-fold with cystine-free, serum-free medium, and incubate at 37oC.
| 20 tests (4 ml) |
50 tests (10 ml) |
100 tests (20 ml) |
|
| Cystine Analog Solution | 40 μl | 100 μl | 200 μl |
|
cystine-free, serum-free medium |
4 ml | 10 ml | 20 ml |
- Prepare CA uptake solution fresh each day. Pre-warm the CA uptake solution in an incubator (37°C)
– cystine uptake into cells may be affected by the temperature of the CA uptake solution.
2. Preparation of Probe solution
Add DMSO to a tube of Fluorescent Probe and dissolve by pipetting and vortex mixing.
| DMSO | |
| 100 tests | 50 μl |
| 20 tests | 10 μl |
- Centrifuge the tube briefly before opening to remove all content from the walls and cap.
Store the Probe solution at −20°C and protect it from light. The Probe solution is stable for 2 months.
3. Preparation of Reducing agent (RA) solution
Add ddH2O to a tube of Reducing Agent and dissolve by pipetting and vortex mixing.
| ddH2O | |
| 100 tests | 250 μl |
| 20 tests | 100 μl |
- Centrifuge the tube briefly before opening to remove all content from the walls and cap.
Store the RA solution at −20°C and protect it from light. The RA solution is stable for 2 months.
General Protocol
For adherent cells
Use a multichannel pipette to reduce the time difference between loading each well.
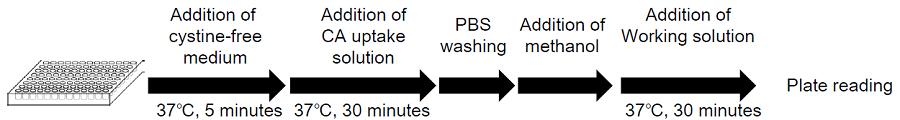
Figure 3. Protocol for adherent cells
- Seed cells in a 96-well microplate and culture the cells at 37°C overnight in a 5% CO2 incubator.
- Remove the culture medium and wash the cells three times with pre-warmed cystine-free, serum-free medium*1.
- Add 200 μl of pre-warmed cystine-free, serum-free medium*1 and incubate at 37°C for 5 minutes in a 5% CO2 incubator.
- Remove the supernatant and add 200 μl of pre-warmed CA uptake solution*1 or pre-warmed cystine-free, serum-free medium*1 (for the blank).
- Incubate at 37°C for 30 minutes in a 5% CO2incubator.
- Remove the supernatant and wash the cells with 200 μl of ice-cold PBS three times.
- Remove the supernatant and add 50 μl of methanol.
- Prepare Working solution.*2
20 tests
(4 ml)50 tests
(10 ml)100 tests
(20 ml)Probe solution 8 μl 20 μl 40 μl RA solution 80 μl 200 μl 400 μl Assay Buffer 4 ml 10 ml 20 ml - Add 200 μl of Working solution, mix by pipetting, and incubate at 37°C for 30 minutes. *3
- Measure the fluorescence intensity using a fluorescence plate reader (Ex/Em = 490/535 nm) *4.
- Subtract the measured value of the blank from the value measured for each sample.
(Option)
If you cannot obtain a difference in fluorescence intensity between samples or with a blank, correct it using the nuclear staining method. Please refer to the FAQ section on the product website for the specific method.
- Pre-warm the culture medium and CA uptake solution in an incubator (37°C). Cystine uptake into cells may be affected by the temperature.
- Prepare Working solution immediately before use.
- When incubating, use a microplate seal to prevent evaporation.
- Normalize the fluorescence values by the number of cells (determined by nuclear staining),as needed.
For suspension cells
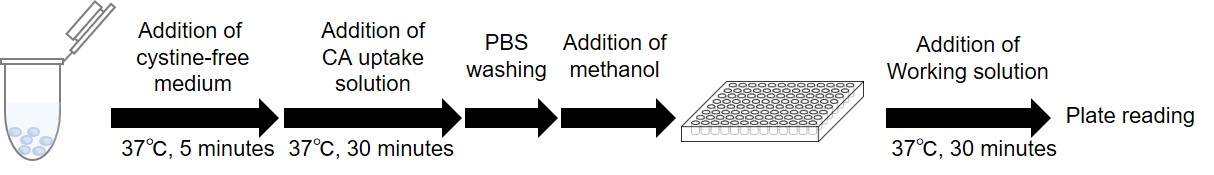
Figure 4. Protocol for suspension cells
- Dispense cells into microtubes. *Example: If you want to measure 2 different samples as triplicate, prepare 9 samples (sample 1, 2, blank: n=3).
- The cells are centrifuged at 300 × g for 3 minutes
- After removing the supernatant, 500 μl of pre-warmed medium (cystine-free serum-free medium ;37℃*1) is added,
the cells are then resuspended and centrifuged at 300 x g for 3 minutes. This step is repeated twice. - After removing the supernatant, 500 μl of pre-warmed medium (cystine-free serum-free medium ;37℃*1) is added,the cells are then resuspend, and incubated at 37℃ for 5 minutes in a 5% CO2 incubator, and centrifuged at 300 x g for 3 minutes.
- After removing the supernatant, 500 μl of pre-warmed CA uptake solution or cystine-free serum-free medium at 37℃ are added. The cells are then resuspended, and incubated at 37℃ for 30 minutes in a 5% CO2 incubator, and centrifuged at 300 x g for 3 minutes.
- After removing the supernatant, 500 μl of ice-cold PBS is added. The cells are then resuspended and centrifuged at 300 x g for 3 minutes. This step is repeated twice.
- After removing the supernatant, 100 μl of methanol is added and mixed by pipetting.
Note: To correct the measured value for protein concentration, use 50 μl of the solution from step 7 for the UP05 measurement and use the remaining portion for the protein concentration measurement. - Prepare Working solution.*2
20 tests
(4 ml)50 tests
(10 ml)100 tests
(20 ml)Probe solution 8 μl 20 μl 40 μl RA solution 80 μl 200 μl 400 μl Assay Buffer 4 ml 10 ml 20 ml - The solution from step 7 (50 μl) is transferred to a 96-well black microplate, and 200 μl of Working solution was added and mixed by pipetting. The plate was sealed and incubated at 37°C for 30 minutes.*3
Note: Thoroughly suspend the solution in step 7 before transferring it to the microplate. - Measure the fluorescence intensity using a fluorescence plate reader (Ex/Em = 490/535 nm) *4.
- Subtract the measured value of the blank from the value measured for each sample.
(Option)
If you cannot obtain a difference in fluorescence intensity between samples or with a blank, correct for the protein concentration. Please refer to the FAQ section on the product website for the specific method.
- ※1 The cystine uptake capacity of cells may be affected by the temperature of the solution. Please warm the solution in an incubator (37°C) in advance.
- ※2 Working solution should be prepared immediately before use.
- ※3 Use a plate seal during incubation to prevent evaporation.
- ※4 If necessary, correct the obtained fluorescence intensity for protein concentration.
Experimental example for adherent cells
Inhibition of xCT activity by xCT inhibitor erastin (HeLa cells)
- HeLa cells (1.5×104 cells/well, 150 μl) in Minimum Essential Medium (MEM; 10% fetal bovine serum) were seeded in a 96-well black microplate (655090, Greiner) and cultured overnight at 37°C in a 5% CO2 incubator.
- After removing the supernatant, the cells were washed three times with 200 μl of pre-warmed Dulbecco’s modified Eagle’s medium (DMEM; cystine-free, serum-free medium, 2 mmol/l glutamine, 21013024: Thermofisher Scientific).
- Pre-warmed DMEM (200 μl, cystine-free, serum-free medium, 2 mmol/l glutamine) containing 0 μmol/l (sample 1 and the blank) or 100 μmol/l erastin (sample 2) was added, and the cells were incubated at 37°C for 5 minutes in a 5% CO2 incubator.
- After removing the supernatant, 200 μl of pre-warmed CA uptake solution containing 0 μmol/l erastin (sample 1), 100 μmol/l erastin (sample 2) or DMEM (cystine-free, serum-free medium, 2 mmol/l glutamine, for the blank) were added, and the cells were incubated at 37°C for 30 minutes in a 5% CO2 incubator.
- After removing the supernatant, the cells were washed three times with 200 μl of ice-cold PBS.
- After removing the supernatant, 50 μl of methanol were added.
- Working solution (200 μl) was added, mixed by pipetting, and incubated at 37°C for 30 minutes. The plate was sealed.
- The fluorescence intensity was measured using a fluorescence plate reader (Ex/Em = 490/535 nm; Infinite m200 PRO, Tecan Trading AG).
- The fluorescence intensity derived from the incorporated Cystine Analog was calculated by subtracting the blank value from the value measured for each sample.
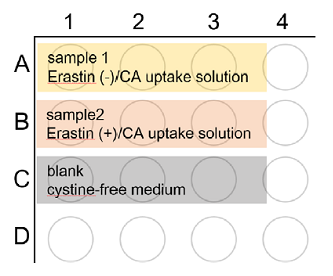
Figure 5. Plate layouts
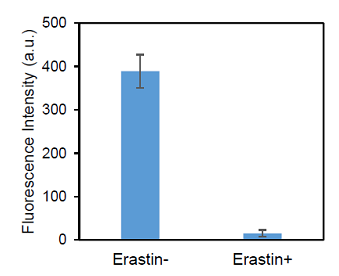
Figure 6. Inhibition of cystine transporter activity in HeLa cells by erastin
Experimental example for suspension cells
Inhibition of xCT activity by erastin (HL60 cells)
- HL60 cells were dispensed into nine tubes at 1.0×106 cells/tube (samples 1, 2, and blank; n = 3).
- The cells were centrifuged at 300 x g for 3 minutes.
- After removing the supernatant, 500 μl of pre-warmed DMEM (cystine-free, serum-free medium, 2 mmol/l glutamine, 21013024: Thermofisher Scientific) were added. The cells were resuspended and centrifuged at 300 x g for 3 minutes.
This step was repeated twice. - Pre-warmed DMEM (500 μl) containing 0 μmol/l (sample 1 and the blank) or 100 μmol/l erastin (sample 2) was added, and the cells were resuspended and incubated at 37°C for 5 minutes in a 5% CO2 incubator.
- The cells were centrifuged at 300 x g for 3 minutes.
- After removing the supernatant, 500 μl of pre-warmed CA uptake solution containing 0 μmol/l erastin (sample 1),100 μmol/l erastin (sample 2) or DMEM (blank) were added. The cells were resuspended and incubated at 37°C for 30 minutes in a 5% CO2 incubator.
- The cells were centrifuged at 300 x g for 3 minutes.
- After removing the supernatant, 500 μl of ice-cold PBS was added. The cells were resuspended and centrifuged at 300 x g for 3 minutes. This step was repeated twice.
- After removing the supernatant, 100 μl of methanol were added and mixed by pipetting.
Note: Used 50 μl of the solution from step 9 for the UP05 measurement and used the remaining portion for the protein concentration measurement. - The solution from step 9 (50 μl) was transferred to a 96-well black microplate, and 200 μl of Working solution was added and mixed by pipetting. The plate was sealed and incubated at 37°C for 30 minutes.
Note: Thoroughly suspend the solution in step 9 before transferring it to the microplate. - The fluorescence intensity was measured using a fluorescence plate reader (Ex/Em = 490/535 nm; Infinite m200 PRO).
- The fluorescence intensity derived from the incorporated Cystine Analog was calculated by subtracting the blank value from the value measured for each sample.
(Fluorescence intensity correction) - RIPA Buffer (50 μl; 16488-34, Nacalai Tesque) was added to the remainder of the solution from step 9 and mixed by vortexing. The mixture was allowed to stand at room temperature for 30 minutes.
- The solution from step 13 (15 μl) was used for protein quantification (by BCA assay), and the fluorescence intensity obtained in step 12 was normalized by the protein concentration.
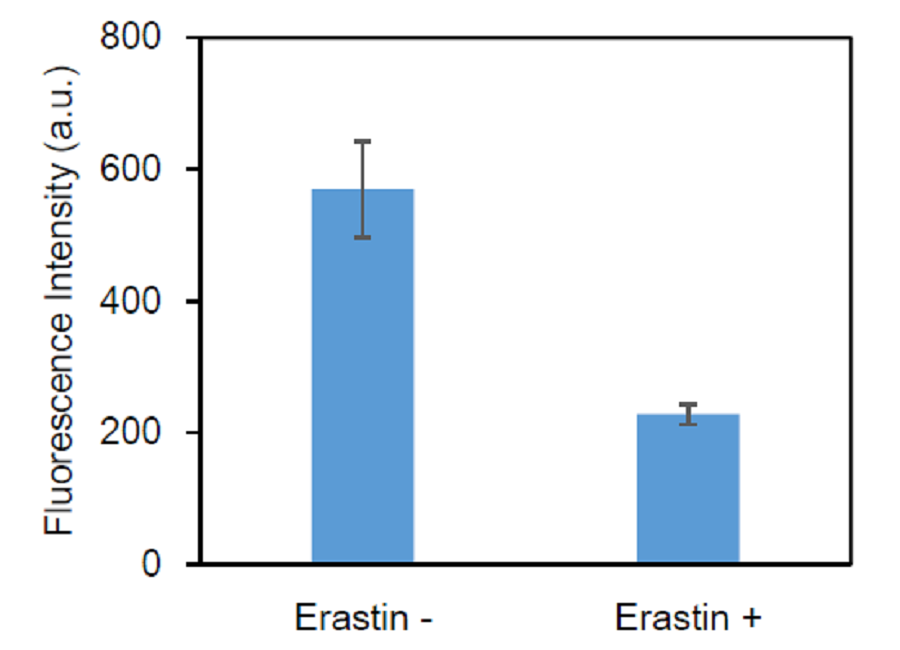
Figure 7. Inhibition of cystine transporter activity in HL60 cells by erastin
Frequently Asked Questions / Reference
UP05: Cystine Uptake Assay Kit
Revised Aug., 18, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.