General Information
Biotin-HPDP(WS) is a novel water-soluble biotin-labeling reagent that is modifi ed from Biotin-HPDP (Product code: B573).
Biotin-HPDP is a conventional reagent to introduce a biotin moiety to a sulfhydryl group of protein via reversible disulfi de bond. This reagent is useful for affi nity-purifi cation of biotinylated proteins by using avidin-coated beads, because the disulfi de bond is cleavable by a reducing agent (Fig.1). Thus, Biotin-HPDP has been widely used for biotin switch assay, which is an analytical technique for protein thiol modifi cations such as s-nitrosylation, s-sulfhydration, and s-palmitoylation. However, preparation of the solution is time-cosuming due to the low-solubility in water, even DMSO and DMF. Therefore, Biotin-HPDP(WS) solution was developed to improve the solubility of Biotin-HPDP.
- SulfoBiotics- Biotin-HPDP(WS) solution is an easy-to-use aqueous solution including 20mmol/l Biotin-HPDP(WS).
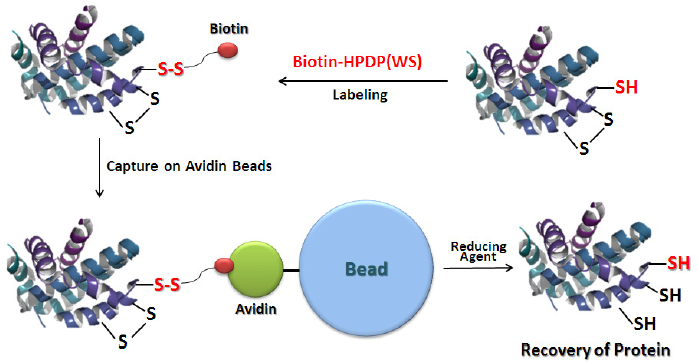
Fig. 1 Schematic Protocol for Biotin Labeling with Biotin-HPDP(WS) and Purifi cation of the Biotinylated Protein
Contents
| 20 mmol/l Biotin-HPDP(WS) solution | 500 μl x 1 |
Storage Conditions
Store at 0-5 ℃
Precautions
Centrifuge the tube briefly before opening the cap because the content may be on the tube wall or on the ceiling of the cap.
Preparation
Dilute the solution with an appropriate buffer or water depending on your experiment.
- Note: Do not use any solutions including reducing agents such as dithiothreitol (DTT) and 2-mercaptoethanol (2ME), because they interfere with biotin labeling.
Experimental Example
Biotin Labeling to GAPDH and the Recovery with Avidin Beads
< Equipment and Materials >
- Incubator (37 ℃)
- 20 µl, 200 µl, 1000 µl pipettes
- Centrifuge
- Multi-imager
- 1.5 ml-microtube
- 10 K Filtration tube
- Lysis buffer : 6 mol/l Urea, 2% SDS, 150 mmol/l Tris-HCl (pH7.4)
- RIPA buffer : 50 mmol/l Tris-HCl (pH8.0), 150 mmol/l NaCl, 0.5% Sodium deoxycholate, 0.1% NP- 40
- NeutravidinTM Agarose (Thermo Fisher Scientific)
- Neutralization buffer : 20 mmol/l HEPES (pH7.7), 100 mmol/l NaCl, 1 mmol/l EDTA, 0.5% TritonX-100
- Neutralization buffer (+600 mmol NaCl) : Neutralization buffer, 600 mmol NaCl
- Elution buffer : 20 mmol/l HEPES (pH 7.7), 100 mmol/l NaCl, 1 mmol/l EDTA, 100 mmol/l 2-Mercaptoethanol
< Procedure >
- Lysis buffer (35 µl) and 100 mmol/l DTT in Lysis buffer (5µl) were added to 10 µl of 1 mg/ml GAPDH solution in PBS in a 1.5 ml-microtube, and mixed by vortex.
- After the solution was incubated at 37 ℃ for 30 minutes, the whole solution was transferred to a 10 K filtration tube, and centrifuged at 12,000 rpm for 10 minutes.
- PBS (50 µl) was added to the fi ltration tube, and centrifuged at 12,000 rpm for 10 minutes.
- Step 3 was repeated.
- RIPA buffer (126 µl) and 4 mmol/l Biotin-HPDP(WS) solution in H2O (14 µl) were added to the filtration tube, and mixed with the protein by pipetting.
- The filtration tube was incubated at 37 ℃ for 1 hour, and centrifuged at 12,000 rpm for 10 minutes.
- PBS (50 µl) was added to the tube, and centrifuged at 12,000 rpm for 10 minutes.
- Step 7 was repeated.
- Neutralization buffer (400 µl) was added to the tube to dissolve the biotin-labeled protein by pipetting, and the solution was transferred to a 1.5 ml-microtube.
- The solution (50 µl) of Step 9 was added to NeutravidinTM Agarose beads in a tube.
- Note: Neutravidin Agarose beads were washed with Neutralization buffer prior to the reaction.
- The solution was incubated at 4 ℃ for 1 hour.
- The tube was centrifuged at 2,500 rpm for 1 minute, and the supernatant was removed using a pipette.
- Neutralization buffer (+600 mmol/l NaCl) (1 ml) was added to the tube and centrifuged at 2,500 rpm for 1 minute, and the supernatant was removed using a pipette.
- Step 13 was repeated twice.
- Neutralization buffer (1 ml) was added to the tube and centrifuged at 2,500 rpm for 1 minute, and the supernatant was removed using a pipette.
- Step 15 was repeated.
- Elution buffer (50 µl) was added to the tube, and mixed by vortex. The solution was incubated at 4 ℃ for 1 hour.
- The tube was centrifuged at 2,500 rpm for 1 minute, and 10 µl of the supenatant was transferred to a 1.5 ml microtube.
- Loading buffer (2 µl) was added to the microtube, and the solution was applied to SDS-PAGE (CBB staining) and western blotting.
-
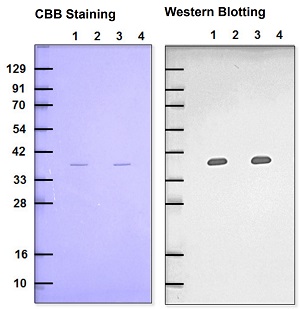
Fig. 2 Detection of recovered GAPDH
(Comparison between Biotin-HPDP and Biotin-HPDP(WS)
-
Lane 1: Biotin-HPDP
Lane 2 : Biotin-HPDP (+NEM blocking)
Lane 3: Biotin-HPDP(WS) solution
Lane 4: Biotin-HPDP(WS) solution (+NEM blocking)
Primary antibody : Rabbit anti-GAPDH antibody
Secondary antibody : Goat anti-Rabbit antibody-POD conjugated Chemiluminescence detection
Biotin-HPDP(WS) showed comparable data with Biotin-HPDP at the same concentrations.
References
- S. R. Jaffrey and Solomon H. Snyder, "The Biotin Switch Method for the Detection of S-Nitrosylated Proteins", Sci. STKE, 2001, 86, pl1.
- X. Wang. N. Kettenhofen, S. Shiva, N. Hogg, and M. Gladwin, "Copper dependence of the biotin switch assay : modified assay for measuring cellular and blood nitrosated proteins", Free Radic. Biol. Med., 2008, 44, 1362.
- M. T. Forrester, M. W. Foster, M. Benhar, and J. S. Stamler, "Detection of Protein S-Nitrosylation with the Biotin Switch Technique", Free Radic. Biol. Med., 2009, 46(2), 119.
- M. D. Kornberg, N. Sen, M. R. Hara, K. R. Juluri, J. V. K. Nguyen, A. M. Snowman, L. Law, L. D. Hester, and S. H. Snyder, "GAPDH Mediates Nitrosylation of Nuclear Proteins". Nat. Cell Biol., 2010, 12(11), 1094.
- J. Wan, A. F. Roth, A. O. Bailey, and N. G. Davis, "Palmitoylated proteins: purification and identification", Nat.Protoc., 2007, 1573.
- A. K. Mustafa, M. M. Gadalla, N. Sen, S. Kim, W. Mu, S. K. Gazi, R. K. Barrow, G. Yang, R. Wang, and S. H.
Snyder, "H2S Signals Through Protein S-Sulfhydration", Sci. Signal., 2009, 2(96), га72.
Frequently Asked Questions / Reference
SB17: -SulfoBiotics- Biotin-HPDP(WS) solution
Revised Apr., 11, 2024


 Hidden sections will not be printed.
Hidden sections will not be printed.

