General Information
Reactive oxygen species (ROS) are mainly produced in mitochondria during ATP synthesis. ROS play an essential role in signaling pathways and the immune system, but excess ROS are associated with diseases and cellular senescence. DCFH-DA is widely used for ROS detection, but it has some limitations, such as weak fluorescence signals in cells, leakage from cells after staining, and photo-oxidation phenomena during observation.
Dojindo’s ROS Assay Kit -Photo-oxidation Resistant DCFH-DA- overcomes these limitations:
The dye that is employed in this kit allows ROS detection with higher sensitivity than DCFH-DA; It does not leak from cells because the fluorescent dye can immobilize protein via a chemical bond; and it is resistant to photo-oxidation compared with DCFH-DA. Moreover, the Loading Buffer included in the kit maintains cellular health during assays.
Kit Contents
| Photo-oxidation Resistant DCFH-DA Dye | 100 nmol×1 |
| Loading Buffer (10x) | 1 ml×1 |
Storage Condition
Store at 0–5℃
Required Equipment and Materials
- Fluorescence microscope, fluorescence plate reader, or flow cytometer
- Incubator (37℃)
- Micropipettes (100–1000 μl, 20–200 μl, 1–10 μl)
- Microtubes
- Hanks' Balanced Salt Solution (HBSS)
- Dimethyl Sulfoxide (DMSO)
Precaution
Allow the ROS Assay Kit -Photo-oxidation Resistant DCFH-DA- to equilibrate at room temperature before use.
Centrifuge the tube briefly before opening the cap because the content may be on the tube wall or the ceiling of the cap.
Please refer to Table 1 to check suitable fluorescence wavelengths for each application.
Table 1. Recommended filter settings
| Applications | Fluorescence plate reader | Fluorescence microscope | Flow cytometer |
| Measurement wavelength | Ex/Em: 490–520/510–540 nm | ・Confocal microscope Ex/Em: 488/500–550 nm ・Fluorescence microscope GFP or FITC filter |
FITC filter |
Preparation of Solutions
Preparation of Loading Buffer Solution
Dilute the 10× Loading Buffer (1:10) with double-deionized water.
- Refer to Table 2 for preparing the Loading Buffer Solution.
Table 2. Required amount of Loading Buffer Solution by vessel type
| Vessel | 35-mm dish | ibidi 8-well plate | 96-well clear black plate (clear bottom) | Flow cytometer |
| Appropriate amount | 2 ml/dish | 200 μl/well | 100 μl/well | 0.5 ml/tube |
- Use the diluted Loading Buffer Solution on the same day.Store the remaining Loading Buffer (10×) at 4℃.
Preparation of Photo-oxidation Resistant DCFH-DA DMSO Stock Solution (10 mmol/l)
Add 10 μl DMSO to the Photo-oxidation Resistant DCFH-DA Dye tube and dissolve by pipetting.
- Photo-oxidation Resistant DCFH-DA Dye is difficult to see because it is a small amount and is a colorless form.
After adding DMSO, repeat pipetting several times to dissolve Photo-oxidation Resistant DCFH-DA Dye.
Store the reconstituted Photo-oxidation Resistant DCFH-DA DMSO Stock Solution at −20℃ until use.
The solution is stable at −20℃ for 1 month.
Preparation of Photo-oxidation Resistant DCFH-DA Working Solution
Dilute the 10 mmol/l Photo-oxidation Resistant DCFH-DA DMSO Stock Solution (1:1000) in reconstituted Loading Buffer Solution to prepare 10 μmol/l Photo-oxidation Resistant DCFH-DA Working Solution.
- The working solution cannot be stored and must be prepared freshly each day.
General protocol
- Seed cells on dishes or plates and place them in an incubator at 37℃ equilibrated with 95% air and 5% CO2 overnight.
- Refer to Table 3 to check the recommended vessels for each detector.
Table 3. Recommended vessels by a detector
Detector Fluorescence plate reader Fluorescence microscope Flow cytometer Vessel 96-well black plate (clear bottom) 96-well black plate (clear bottom)
ibidi 8-well plate
35-mm dish6-well plate - Discard the culture medium and wash the cells twice with HBSS.
- Discard the supernatant and add an appropriate volume of Photo-oxidation Resistant DCFH-DA Working Solution to the cells. Incubate the cells as in step 1 for 30 minutes.
- Discard the working solution and wash the cells twice with HBSS.
- Discard the supernatant and add a medium containing ROS-inducing agent and incubate as in step 1 for an appropriate time.
- Discard the supernatant, wash the cells twice with HBSS, add HBSS, and observe fluorescence signals.
- Perform steps 3 and 6 in the reverse order in case dye staining after ROS-inducing is needed.
- Adjust the incubation time to allow the cells to adhere completely.
Usage examples
Detection of ROS in RAW264.7 cells treated with lipopolysaccharide (LPS).
- RAW264.7 cells (2×105 cells/ml) in DMEM (supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin) were seeded into vessels and incubated overnight at 37℃ in 95% air/5% CO2.
- After the culture medium was removed, DMEM containing LPS (500 ng/ml) was added to the cells.
- The cells were cultured for 24 hours as in step 1.
- The supernatant was removed, and the cells were washed twice with HBSS.
- After removing the supernatant, Photo-oxidation Resistant DCFH-DA Working Solution was added and the cells were cultured for 30 minutes as in step 1.
- The cells were washed twice with HBSS, and the vessels were refilled with HBSS.
- Fluorescence signals were measured using a plate reader and a flow cytometer, and imagings was performed by fluorescence microscopy.
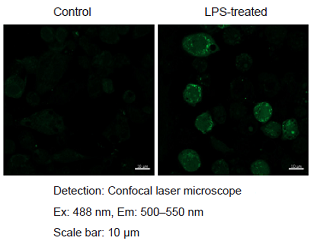
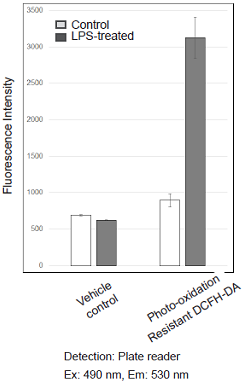
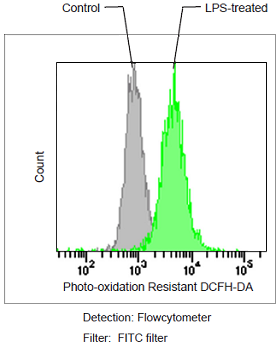
Figure 1. Fluorescence signals from RAW264.7 cells detected with a confocal laser microscope, flow cytometer and plate reader
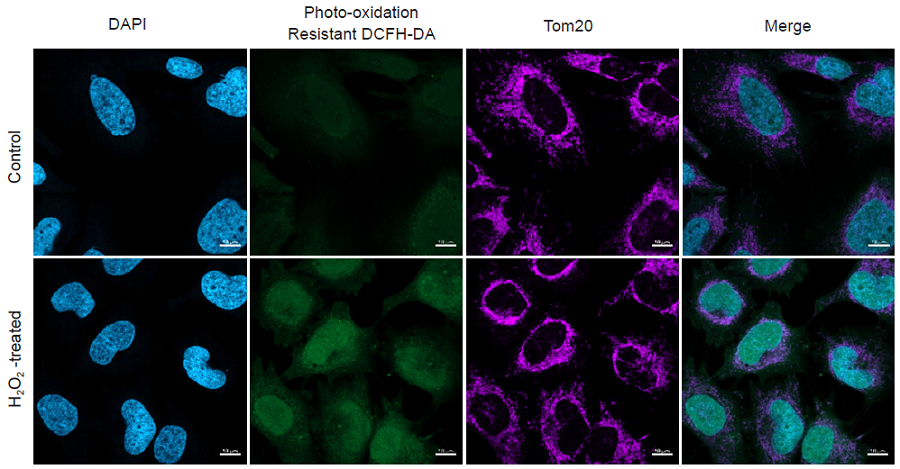
Multiple staining of HeLa cells with DAPI, Photo-oxidation Resistant DCFH-DA and anti-Tom20 antibody as a mitochondrial marker.
- HeLa cells (2×105 cells/ml) in MEM (supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin) were seeded on ibidi 8-well plates and cultured overnight at 37℃ in 95% air/5% CO2.
- The medium was removed, and the cells were washed twice with HBSS.
- The cells were stained with Photo-oxidation Resistant DCFH-DA Working Solution and incubated for 30 minutes as in step 1.
- After removing the supernatant, and the cells were washed twice with HBSS.
- The supernatant was removed, 100 μmol/l hydrogen peroxide in HBSS was added to each well, and the cells were cultured for 30 minutes as in step 1.
- Removed the supernatant, and the cells were washed twice with HBSS.
- The supernatant was replaced with a paraformaldehyde (4% v/v) in phosphate-buffered saline (PBS), and the cells were incubated at room temperature for 15 minutes.
- After removing the supernatant, the cells were washed three times with PBS.
- The supernatant was replaced with a Triton X-100 (0.1% v/v) in PBS, and the cells were incubated at room temperature for 10 minutes.
- Removed the supernatant, and the cells were washed three times with PBS.
- After removing the supernatant, a blocking solution was added, and the cells were incubated at room temperature for 20 minutes.
- After removing the supernatant, an anti-Tom20 antibody (1 μg/ml) in blocking solution was added, and the cells were incubated at 4℃ overnight.
- Removed the supernatant, and the cells were washed three times with PBS-T.
- The supernatant was replaced with an Alexa647 labeled anti-mouse igG (2 μg/ml) and DAPI (1 μg/ml) in a blocking solution, and the cells were incubated at room temperature for 1 hour.
- After removing the supernatant, the cells were washed twice with PBS-T.
- The cells were observed under a confocal laser scanning microscope (LSM 800, Zeiss).
- blocking solution: 10% (v/v) Blocking One in PBS-T
|
Detection: Confocal laser microscope |
Figure 2. Simultaneous fluorescence imaging of nucleus, hydrogen peroxide and mitochondria in HeLa cells
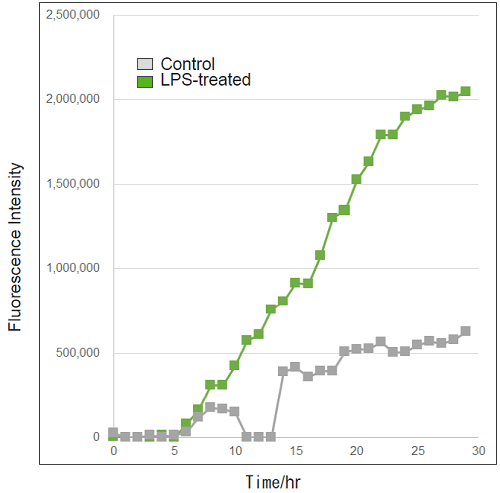
Detection of ROS in RAW264.7 cells treated with LPS (timelapse imaging).
- RAW264.7 cells (2×105 cells/ml) in DMEM (supplemented with 10% fetal bovine serum and 1% penicillin-streptomycin) were seeded on a 96-well black clear-bottomed plate and incubated overnight at 37℃ in 95% air/5% CO2.
- The medium was removed, and the cells were washed twice with HBSS.
- The cells were treated with Photo-oxidation Resistant DCFH-DA Working Solution and incubated for 30 minutes as in step 1.
- After removing the supernatant, the cells were washed twice with HBSS.
- After removing the supernatant, DMEM containing LPS (500 ng/ml) was added to the cells.
- The cells were observed every hour for 30 hours under an epifluorescence microscope (BZ-X800, KEYENCE).
- Fluorescence signals were quantified.
|
Detection: Epifluorescence microscope |
Figure 3. Fluorescence signals from RAW264.7 cells over time
Reference
- M. Afzal, et al., BBRC, 2003, 304, 619–624
Frequently Asked Questions / Reference
R253: ROS Assay Kit -Photo-oxidation Resistant DCFH-DA-
Revised Feb., 02, 2025


 Hidden sections will not be printed.
Hidden sections will not be printed.

