General Information
Cells have intracellular organelles that function to produce energy, synthesize and degrade proteins. Typical organelles include the nucleus, mitochondria, endoplasmic reticulum, and lysosomes. Although research on the functions of these organelles has been actively conducted for some time, recent developments in imaging technology, such as super-resolution imaging, have revealed that not only the functions of each organelle but also the coordination among organelles is important. One such example is the mitochondria-associated endoplasmic reticulum membrane, which is the interface between mitochondria and the endoplasmic reticulum (ER). Visualization of mitochondria is necessary to observe the interaction between mitochondria and other organelles. Fluorescent protein labeling, immunostaining, and mitochondria-selective small molecule fluorochromes are commonly used for this purpose, although these methods have problems. The fluorescent protein labeling method requires gene transfection, and multiple staining may not be able to perform in the immunostaining method depending on the animal species of the primary antibody. In addition, commercially available mitochondria-selective small molecule fluorescent dyes do not have good retention properties and are difficult to use in immunostaining.
To overcome the problems above, Dojindo has developed a new mitochondria-selective small-molecule fluorescent dye, MitoBright IM Red, which covalently binds to proteins and other biomolecules. MitoBright IM Red, therefore, is able to simultaneously perform the staining mitochondria and the immunostaining.
Contents
| 1 Tube | 3 Tubes | |
| MitoBright IM Red for Immunostaining | 20 μl x 1 | 20 μl x 3 |
Storage Condition
Store at -20°C
Required Equipment and Materials
- Growth medium or HBSS (Hanks balanced salt solution)
- Micropipettes
Preparation of Solutions
Preparation of MitoBright IM Red working solution
Dilute the 1 mmol/l MitoBright IM Red solution with growth medium (serum-free) to prepare a 1 μmol/l MitoBright IM Red working solution.
- The MitoBright IM Red working solution cannot be stored and must be freshly prepared each day and used within the day.
General protocol
- Seed cells on a dish. Culture the cells at 37°C overnight in a 5% CO2 incubator.
- Discard the supernatant and wash the cells once with growth medium or HBSS.
- Add the 1 μmol/l MitoBright IM Red working solution to the dish and incubate at 37°C for 30 minutes in a 5% CO2 incubator.
- Discard the supernatant, and wash the cells once with growth medium or HBSS.
- Perform immunofluorescence staining.
- Observe the cells under a fluorescence microscope.
Usage Examples
Multiple staining of MitoBright IM Red with Tom20 as a mitochondrial marker in HeLa cells.
- HeLa cells (2.4 × 104 cells/well, 200 μl) in MEM (10% FBS) were seeded in a μ-slide 8 well plate and cultured at 37°C overnight in a 5% CO2 incubator.
- After removing the supernatant, the cells were washed once with 200 μl of MEM.
- MitoBright IM Red working solution (1 μmol/l, 200 μl) in MEM was added, and the cells were incubated at 37°C for 30 minutes in a 5% CO2 incubator.
- After removing the supernatant, the cells were washed twice with 200 μl of MEM.
- Paraformaldehyde (4% w/v) in PBS (200 μl) was added, and the cells were incubated at room temperature for 15 minutes.
- After removing the supernatant, the cells were washed three times with 200 μl of PBS.
- Triton X-100 (0.1% v/v) in PBS (200 μl) was added, and the cells were incubated at room temperature for 10 minutes.
- After removing the supernatant, 10% (v/v) Blocking One-P in PBS (200 μl) was added, and the cells were incubated at room temperature for 1 hour.
- After removing the supernatant, anti-Tom20 antibody (1 μg/ml, 200 μl) was added, and the cells were incubated at room temperature for 1 hour.
- After removing the supernatant, the cells were washed three times with 200 μl of PBS.
- Alexa647 labeled anti-mouse IgG (2 μg/ml, 200 μl) was added, and the cells were incubated at room temperature for 1 hour.
- After removing the supernatant, the cells were washed twice with 200 μl of PBS.
- The cells were observed under a confocal laser scanning microscope (LSM 800, Zeiss).
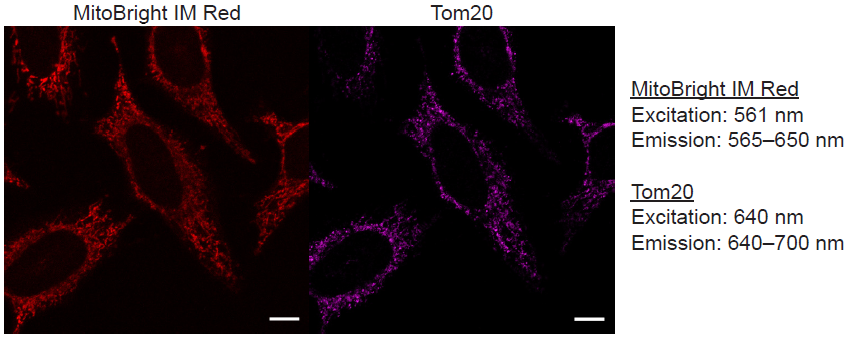
Figure 1 Fluorescence images using MitoBright IM Red and Tom20.
Multiple staining of MitoBright IM Red with KDEL as an ER marker in HeLa cells.
- HeLa cells (2.4 × 104 cells/well, 200 μl) in MEM (10% FBS) were seeded in a μ-slide 8 well plate and cultured at 37°C overnight in a 5% CO2 incubator.
- After removing the supernatant, the cells were washed once with 200 μl of MEM.
- MitoBright IM Red working solution (1 μmol/l, 200 μl) in MEM was added, and the cells were incubated at 37°C for 30 minutes in a 5% CO2 incubator.
- After removing the supernatant, the cells were washed twice with 200 μl of MEM.
- Paraformaldehyde (4% w/v) in PBS (200 μl) was added, and the cells were incubated at room temperature for 15 minutes.
- After removing the supernatant, the cells were washed three times with 200 μl of PBS.
- Triton X-100 (0.1% v/v) in PBS (200 μl) was added, and the cells were incubated at room temperature for 10 minutes.
- After removing the supernatant, 10% (v/v) Blocking One-P in PBS (200 μl) was added, and the cells were incubated at room temperature for 1 hour.
- After removing the supernatant, anti-KDEL antibody (1 μg/ml, 200 μl) was added, and the cells were incubated at 4°C for overnight.
- After removing the supernatant, the cells were washed three times with 200 μl of PBS.
- Alexa488 labeled anti-mouse IgG solution (2 μg/ml, 200 μl) was added, and the cells were incubated at room temperature for 1 hour.
- After removing the supernatant, the cells were washed twice with 200 μl of PBS.
- The cells were observed under a confocal laser scanning microscope (LSM 800, Zeiss).
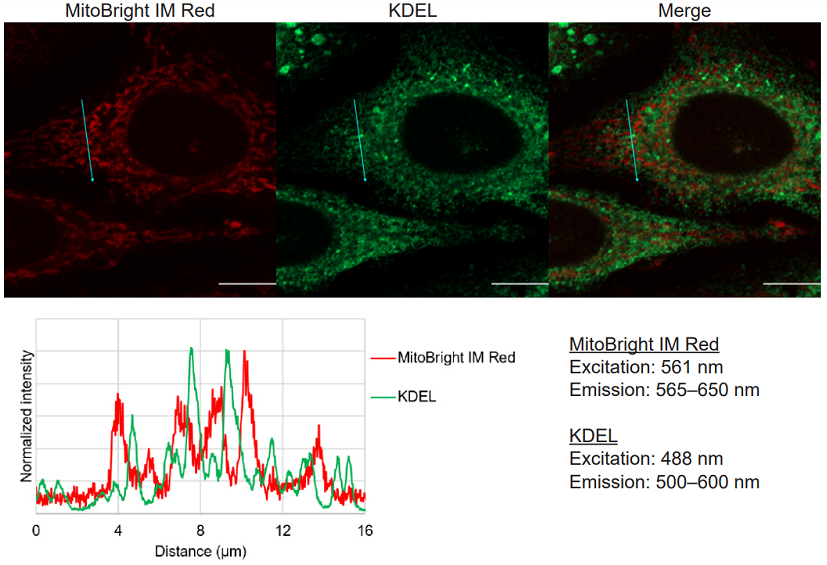
Figure 2 Multiple staining images and line profiles of MitoBright IM Red and KDEL.
Supplemental Information
Excitation and emission spectra of MitoBright IM Red
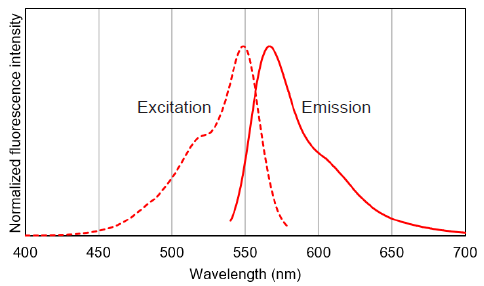
Figure 3 Excitation and emission spectra of MitoBright IM Red.
Frequently Asked Questions / Reference
MT15: MitoBright IM Red for Immunostaining
Revised May., 31, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.