General Information
Ab-10 Rapid Fluorescein Labeling Kit is rapid (in less than 30 min) and easy preparation kit of fluorescein-labeled antibody (Ab) for 10 μg antibody. Reactive Fluorescein (a component of the kit) has succinimidyl ester groups, that can easily make a covalent bond with an amino group of the target antibody without any activation process. This kit contains all the necessary reagents to prepare a fluorescein-labeled antibody.
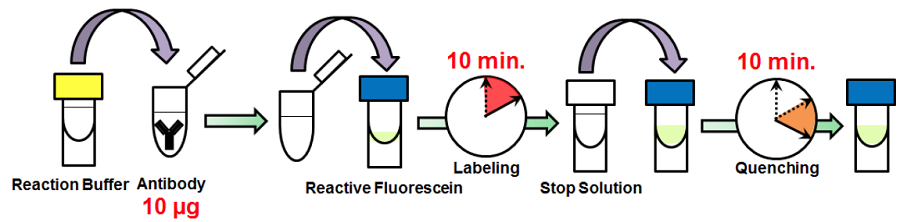
Fig. 1 Labeling procedure
|
Caution After a Reactive Fluorescein is taken out from the seal bag, keep the unused Reactive Fluorescein(s) in the bag, seal tightly and store at -20°C. Store the other components at 0-5°C. |
Kit Contents
| Reactive Fluorescein | x 3 |
| Reaction Buffer | 100 μl x 1 |
| Stop Solution | 100 μl x 1 |
Storage Condition
Store at 0-5 ℃
This kit is stable for 1 year at 0-5℃ before opening.
Required Equipment and Materials
- 20 μl adjustable pippet
- Microtube (for sample preparation)
- Incubator (37°C)
- PBS (Phosphate buffered saline)
Precaution
- Use 0.5-1 mg/ml of antibody solution for labeling. If the antibody concentration is more than 1 mg/ml, please dilute the antibody solution with PBS.
- If the sample solution contains small insoluble materials, centrifuge the solution, and use the supernatant for the labeling.
- The microtubes in this kit contain solutions. Since there is a possibility that the droplets might attach to the inside walls or caps, please spin down to drop them down prior to open.
- Some additives in an antibody solution may interfere with the labeling if the concentration is too high. The maximum compatible concentrations of such additives are indicated in Table 1.
|
|
||||||||||||||||||||||||
- Containing BSA may result in non-specific signal depending on the antibodies used. Removing BSA prior to the fluorescein labeling is recommended in case high non-specific signal is observed.
Protocol
- Add 0.5-1 mg/ml of the antibody solution to a microtube to be an amount of antibody of 10 μg.
- Add Reaction Buffer to the antibody solution (step 1) and mix by pipetting.
- The volume of Reaction Buffer: one-tenth of the antibody solution (Table 2).
- Add the solution (step 2) to Reactive Fluorescein and mix by pipetting.
- Incubate at 37°C for 10 minutes.
- Add Stop Solution to the solution (step 4) and mix by pipetting.
- The volume of Stop Solution: one-tenth of the antibody solution (Table 2).
- Incubate at room temperature for 10 minutes.
- Apply the sample (step 6) for desired experiments or store at 0-5°C.
- The labeled antibody is stable at 4°C for 2 weeks. For longer storage, add equal volume of glycerol to the sample solution and store at -20°C.
| The concentration of antibody (mg/ml) |
0.5 | 0.6 | 0.7 | 0.8 | 0.9 | 1.0 |
|---|---|---|---|---|---|---|
| The volume of Reaction Buffer(μl) |
2.00 | 1.67 | 1.43 | 1.25 | 1.11 | 1.00 |
| The volumeof Stop Solution(μl) |
2.00 | 1.67 | 1.43 | 1.25 | 1.11 | 1.00 |
Supplimental Information
Mitochondria immunostaining
- HeLa cells were seeded on a μ-slide 8 well (ibidi) and cultured overnight at 37 ℃ in a 5% CO2 incubator.
- The cells were washed using PBS three times, and 4% paraformaldehyde in PBS was added to the μ-slide.
- The cells were then incubated at room temperature for 1 hour.
- The supernatant was discarded and 1% Triton-X in PBS was added to the μ-slide.
- The μ-slide was incubated at room temperature for 30 minutes.
- Once the cells were washed with PBS three times, a blocking solution prepared with PBS was added to the μ-slide.
- The cells were then incubated at 0-5℃ for 1 hour.
- Fluorescein conjugated anti-mitochondria antibody was diluted 50 times with the blocking solution.
- Anti-mitochondria antibody was purchased from Abcam (Product Code: ab3298).
- The supernatant was discarded and the solution (step 8) was added to the μ-slide.
- The μ-slide was incubated at 0-5℃ overnight.
- After the cells were washed using Tris buffer (TB, 50 mmol/l, pH 7.5) three times, TB was added to the μ-slide.
- The cells were observed under a fluorescence microscope.
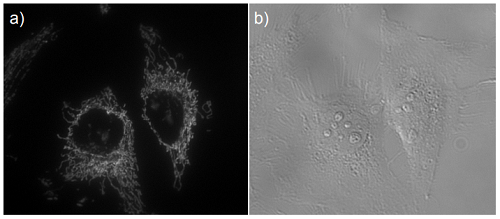
Fig. 2 Microscope image of mitochondria in HeLa cells
a) Fluorescence image, b) Bright field image
Frequently Asked Questions / Reference
LK32: Ab-10 Rapid Fluorescein Labeling Kit
Revised May., 18, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.