General Information
The lysosome is an organelle in which an acid vacuole is formed by a biomembrane. Lysosomes contain various degrading enzymes and contribute to maintaining intracellular homeostasis by acting as a waste disposal system. Recent findings reveal that lysosomal dysfunction is related to some neurodegenerative disorders. Consequently, the investigation of lysosomal function is attracting considerable interest in the scientific community.
This kit includes lysosome staining dyes, pHLys Red (pH dependent) and LysoPrime Green (pH-independent). pHLys Red and LysoPrime Green accumulate in the intact lysosomes. The fluorescence intensity of pHLys Red is enhanced as the acidity increases, and weak fluorescence is observed when lysosomes are neutralized due to the lysosomal dysfunction. On the other hand, LysoPrime Green gives stable emissions even lysosomes are neutralized. Lysosomal pH and lysosomal mass can be measured by combining these two dyes.
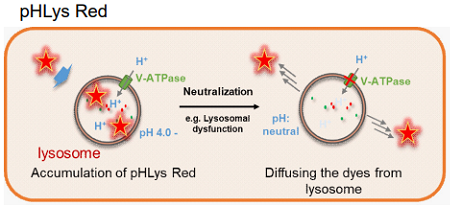
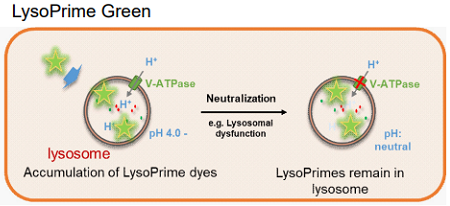
Fig 1. The difference between pHLys Red (pH-dependent) and LysoPrime Green (pH-independent)
Fluorescent Property
Excitation and emission spectra of pHLys Red and LysoPrime Green
-
pHLys Red
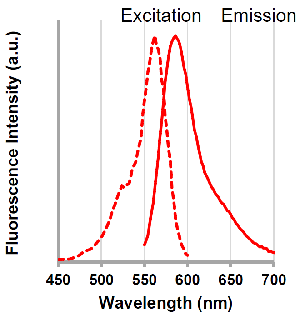
λex: 562 nm
λem: 586 nm
<Filter setting>
Ex : 561 nm
Em : 560 – 650 nm
-
LysoPrime Green
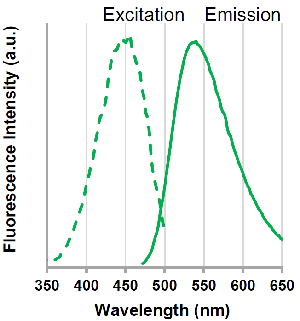
λex: 456 nm
λem: 540 nm
<Filter setting>
Ex : 488 nm
Em : 500 – 600 nm
Contents
| pHLys Red | x 1 |
| LysoPrime Green | 10 μl x 1 |
| Bafilomycin A1 | x 1 |
- pHLys Red and Bafilomycin A1 in each tube may be barely visible due to the small amount. Please handle it carefully.
Storage Condition
Store at -80 °C and protect from light.
- The influence of storage temperature (25℃、4℃、 -20℃、-80℃) on the staining ability of LysoPrime Green is described in the FAQ on our website (product code: L261).
Required Equipment and Materials
- Growth medium
- Hanks’ Balanced Salt Solution (HBSS) or serum-free medium
- Micropipettes
- Microtubes
Preparation of Solution
Preparation of pHLys Red DMSO stock solution
Add 20 μl of DMSO to the provided tube containing pHLys Red, and dissolve by vortex mixer.
- Store the reconstituted pHLys Red DMSO stock solution at −20°C until use. The solution is stable at −20°C for 1 month.
Preparation of pHLys Red working solution
Dilute the pHLys Red DMSO stock solution 1,000 times with growth medium or HBSS to prepare pHLys Red working solution.
- The final concentration of pHLys Red should be optimized depending on the cell line (dilution range: 250 – 1,000 times).
- pHLys Red working solution should be used on the day it is prepared
Preparation of LysoPrime Green working solution
Dilute the LysoPrime Green solution 2,000 times with HBSS or serum-free medium to prepare LysoPrime Green working solution.
- The final concentration of LysoPrime Green should be optimized depending on the cell line (dilution range: 1,000 – 4,000 times).
- Use HBSS or serum-free medium for the dilution because serum in medium interferes with LysoPrime Green.
- LysoPrime Green working solution should be used on the day it is prepared.
Preparation of Bafilomycin A1 DMSO stock solution
Add 24 μl of DMSO to a tube of Bafilomycin A1 and dissolve it with pipetting.
- The solution is stable at −20°C for 1 month.
Preparation of Bafilomycin A1 working solution
Dilute the Bafilomycin A1 DMSO stock solution 2,000 times with HBSS to prepare the Bafilomycin A1 working solution.
- The final concentration of Bafilomycin A1 should be optimized depending on the cell line (dilution range: 1,000 – 5,000 times).
- Bafilomycin A1 working solution should be used on the day it is prepared.
General Protocol
- Seed cells in a dish and culture them overnight at 37 °C in an incubator equilibrated with 95% air and 5% CO2.
- Discard the culture medium and wash the cells twice with serum-free medium or HBSS.
- Add LysoPrime Green working solution to the dish containing the cells and incubate them for 30 minutes at 37 °C in an incubator equilibrated with 95% air and 5% CO2.*1
- Discard the supernatant and wash the cells twice with HBSS.*2
- Add pHLys Red working solution (HBSS) to the dish, and incubate them for 30 minutes at 37 °C in an incubator equilibrated with 95% air and 5% CO2.*3
- Discard the supernatant and wash the cells twice with HBSS or growth medium.
- Add growth medium to the dish, then observe the stained cells under a fluorescence microscope.
- Please be sure to stain LysoPrime Green before drug stimulation.
- Please be sure to stain pHLys Red after staining with LysoPrime Green.
- For stimulation with Bafilomycin A1, please add Bafilomycin A1 to pHLys Red working solution, or Bafilomycin A1 stimulation after staining with pHLys Red.
Usage Example
Observation of the lysosomal pH change by confocal fluorescence microscope
- HeLa cells were seeded (1.0 × 104 cells/well) on a μ-slide 8 well plate (ibidi) and cultured overnight at 37 °C in an incubator equilibrated with 95% air and 5% CO2.
- After washing twice with HBSS, 200 μl of LysoPrime Green working solution (2,000 times dilution) and the cells were incubated at 37 °C for 30 min.
- The supernatant was discarded, and the cells were washed twice with HBSS.
- Two hundred (200 μl) of pHLys Red working solution (HBSS, 1,000 times dilution) containing Bafilomycin A1 (1,000 times dilution), an inhibitor of lysosomal acidification, was added to the plate, and the cells were incubated at 37 °C for 30 min.
- The supernatant was discarded, and the cells were washed twice with HBSS.
- MEM (200 μl, containing 10% fetal bovine serum) was added to the well, and the stained cells were observed under a confocal fluorescence microscope.
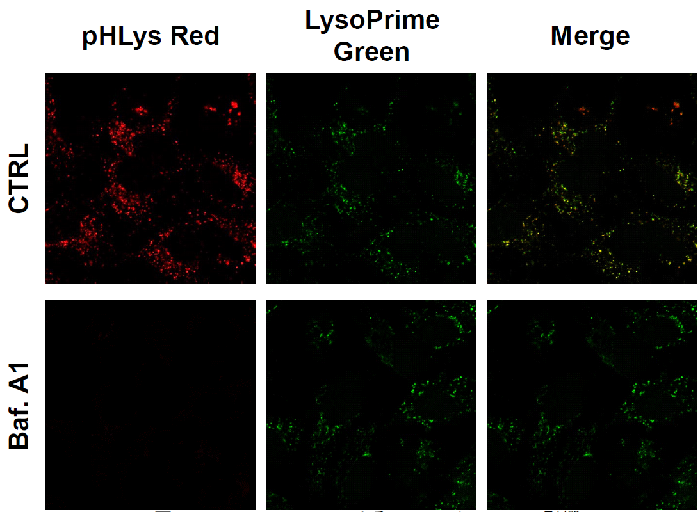
Figure 2. The effect of Bafilomycin A1 on lysosomal pH
| CTRL: Normal condition, Baf. A1: Inhibition of lysosomal acidification pHLys Red filter sets: 561 nm (Ex), 560 – 650 nm (Em) LysoPrime Green filter sets: 488 nm (Ex), 500 – 570 nm (Em) |
Frequently Asked Questions / Reference
L266: Lysosomal Acidic pH Detection Kit
Revised May., 24, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.