General Information
Autophagy is a degradation process of cytoplasmic dysfunctional proteins and organelles. In this process, an isolation membrane composed of a double membrane appears in cytosol, expands gradually, enfolds with the aggregated proteins and damaged organelles, and closes to form autophagosomes. The autophagosomes are fused with lysosomes to form autolysosomes, in which an acidic environment exists. The contents in autolysosomes are decomposed by digestive enzymes in lysosomes. Since this cellular function is said to be related to aging as well as neurodegenerative diseases such as Parkinson’s disease, a simple autophagy detection method which applicable for drug screening has been demanded.
DAPGreen, a small fluorescent molecule, detects autophagosomes and autolysosomes possibly by a mechanism that the dye is incorporated into autophagosome during double membrane formation due to structural features, and then emits fluorescence under hydrophobic conditions. DAPGreen is cell permeable, has no requirement of transfection method, and enables live cell imaging with fluorescence microscopy and quantitative assay by flow cytometry. For monitoring autolysosome, DALGreen [D675] is recommend since it allows detection of phagosomelysosome fusion.
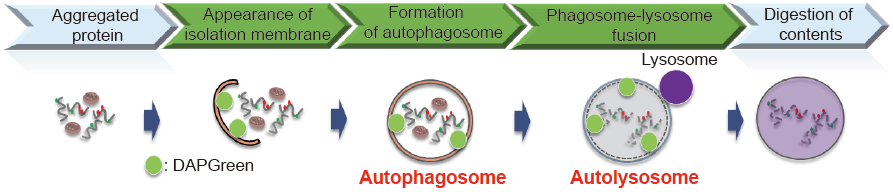
Fig. 1 The detection of autophagy with DAPGreen
Content
| DAPGreen - Autophagy Detection | 5 nmol x 1 |
Storage Condition
Store at -20°C and protect from light.
Required Equipment and Materials
- Dimethyl sulfoxide (DMSO)
- Culture medium
- HBSS or serum-free medium
- Micropipettes
Preparation of Solutions
Preparation of 0.1 mmol/l DAPGreen DMSO stock solution
Add 50 μl of DMSO to a tube of DAPGreen (5 nmol) and dissolve it with pipetting.
- Store the reconstituted DMSO solution at -20°C and protect it from light. The solution is stable at -20°C for 1 month.
Preparation of DAPGreen working solution
Dilute the 0.1 mmol/l DAPGreen DMSO stock solution with culture medium to prepare 0.1-0.5 μmol/l DAPGreen
working solution.
- Please optimize the final concentration of DAPGreen depeneding on the cell lines.
General Protocol
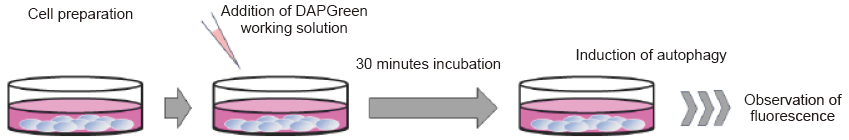
Autophagy detection
- Prepare cells on a dish for assay.
- Discard the supernatant and wash the cells with culture medium.
- Add an appropriate volume of DAPGreen working solution and then incubate at 37°C for 30 minutes.
- Discard the supernatant and wash the cells with culture medium twice.
- Add medium containing autophagy-inducing agent and incubate at 37°C.
- Please optimize the incubation time according to autophagy-inducing conditions.
- Observe fluorescence under a fluorescence microscope or using a flow cytometer.
| Type of instruments | Excitation wavelength (nm) | Emission wavelength(nm) |
| Fluorescent microscope | 425-475 | 500-560 |
| Flow cytometer | 488 | 500-560 |
Experimental Example
Observation by Confocal Fluorescence Microscopy
HeLa cells were seeded on μ-slide 8 well (Ibidi) and cultured at 37°C overnight in a 5% CO2 incubator. The cells were washed with culture medium and then incubated at 37°C for 30 minutes with 250 μl of 0.1 μmol/l DAPGreen working solution. Once the cells were washed with the culture medium twice, the culture medium or amino acidfree medium the (Wako Pure Chemical Industries, Ltd., Code: 048-33575) was added to the well. After 5 hours of incubation, the cells were washed with HBSS twice and then fl uorescence was observed by confocal fl uorescence microscopy.
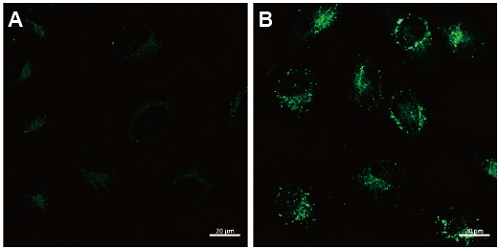
Fig. 2 Confocal microscopic images of HeLa cells staining with DAPGreen (0.1 μmol/l).
The cells were cultured with nutrient-rich medium (A) or with amino acid-free medium (B). Fluorescence images were obtained by confocal microscopy at an excitation wavelength of 488 nm and a 500-563 nm emission fi lter. Scale bar: 20 μm.
Analysis by Flow Cytometry
HeLa cells were seeded on 6 well plate and cultured at 37°C overnight in a 5% CO2 incubator. The cells were washed with culture medium and then incubated at 37°C for 30 minutes with 0.1 μmol/l DAPGreen working solution. After the cells were washed with the culture medium twice, the culture medium or amino acid-free medium was added to the well. After 3 hours of incubation, the cells were washed with PBS, trypsinized and centrifuged. The pellets were suspended in HBSS, and analyzed by flow cytometry.
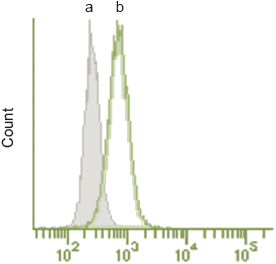
Fig. 3 Detection by flow cytometry.
The cells were cultured with nutrient-rich medium (a) or with the amino acid-free medium (b).
(Ex. 488 nm, Em. 530/30 nm)
Supplemental Information
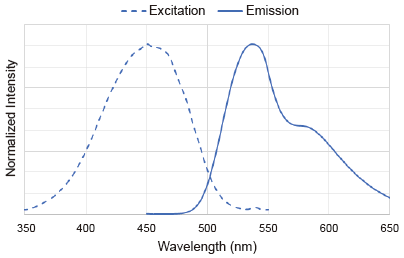
Excitation and emission spectra of DAPGreen
Reference
- H. Iwashita, H. T. Sakurai, N. Nagahora, M. Ishiyama, K. Shioji, K. Sasamoto, K. Okuma, S. Shimizu, and Y. Ueno, ‘’Small fl uorescent molecules for monitoring autophagic fl ux’’, FEBS Lett., 2018, 592, 559-567.
Frequently Asked Questions / Reference
D676: DAPGreen - Autophagy Detection
Revised Aug., 31, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.