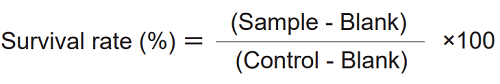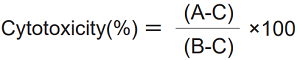General information
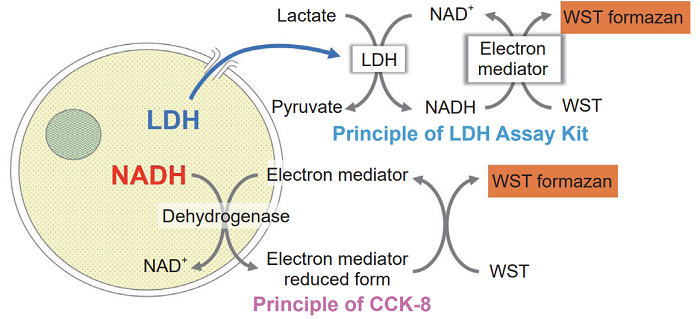
A method for measuring the number of viable cells or that of dead cells is widely used as a cytotoxicity evaluation. In order to determine the cytotoxicity comprehensively, a toxicity evaluation by combining a plurality of evaluation methods has been increasingly demanded. Viability/Cytotoxicity Multiplex Assay Kit can be performed as a comprehensive cytotoxicity assay. Viability/Cytotoxicity Multiplex Assay Kit includes Cell Counting Kit-8 (CCK-8) and Cytotoxicity LDH Assay Kit-WST (LDH Assay Kit), which measures the number of viable cells and dead cells, respectively.
CCK-8 can determine the number of viable cells by measuring dehydrogenases activity in living cells and LDH Assay Kit can determine the number of dead cells by measuring the amount of released LDH from damaged cell membranes. LDH Assay Kit enables both homogeneous assay by adding the reagent solution in the kit to a cell culture medium directly and non-homogeneous assay by adding the reagent solution to a cell culture supernatant. Two index cytotoxicity evaluations with same cultured cells can be carried out by combining CCK-8 and the non-homogeneous assay in LDH Assay Kit.
Kit Contents
| Code | Unit size | Components | ||
| Cell Counting Kit-8 | CK04 | 500 tests | Cell Counting Kit-8 | 5 ml × 1 |
| Cytotoxicity LDH Assay Kit | CK12 | 500 tests | Dye Mixture | × 1 |
| Assay Buffer | 55 ml × 1 | |||
| Lysis Buffer | 5.5 ml × 1 | |||
| Stop Solution | 27.5 ml × 1 | |||
Storage Condition
Store at 0-5℃
Required Equipment and Materials
- Microplate reader with 450 nm and 490 nm filter
- CO2 incubator
- 20, 100-200 μl 8-channel pipette
- 96-well tissue culture plate (flat-bottomed)
- Round or V-bottomed plate for suspension cells in non-homogeneous assay.
- 96-well optically clear plate (flat-bottomed)
- For non-homogeneous assay in LDH measurement.
Precaution
The optimum conditions depend on types of cells. We recommend carrying out a preliminary experiment to optimize the concentration of cells and the incubation time.
Contents of Technical Manual
| CCK-8 assay for viable cell detection |
|
| LDH Assay for cell death detection |
|
・Homogeneous assay
|
・Non-homogeneous assay
|
| Experimental example for CCK-8 and LDH assay in same cells |
- Cell viability and cytotoxicity assay
Serial dilution procedure
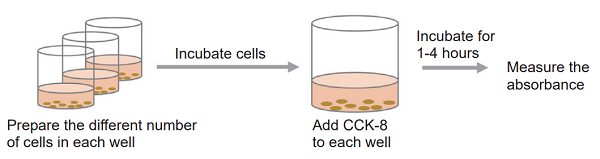
CCK-8 assay for viable cell detection CCK-8 Assay
The ideal number of cells and the incubation time for color development should be determined by a preliminary experiment. Prepare cells without addition of test substances in culture medium.
Optimization of Cell Concentration and Incubation Time
- Collect cells and wash them with the medium. Prepare 5×105 cells/ml cell suspension in the medium.
- Prepare 100 μl of 2-fold serially diluted cell suspension in a 96-well tissue culture plate.
- Refer the Serial Dilution Procedure.
- Incubate cell suspension at 37°C for an appropriate time in a CO2 incubator.
- Add 10 μl of CCK-8 solution to each well of the plate.
- Be careful not to introduce bubbles to the wells, since they interfere the O.D. reading.
- Incubate the plate at 37°C for 1 - 4 hours in the CO2 incubator.
- Color intensity depends on types of cells. Measure the absorbance every hour and find the optimum incubation time which allows absorbance to be in following range.
for cytotoxicity assay : 0.5 - 1.5
for proliferation assay : 0.3 - 0.8
- Color intensity depends on types of cells. Measure the absorbance every hour and find the optimum incubation time which allows absorbance to be in following range.
- Measure the absorbance at 450 - 490 nm by using a microplate reader.
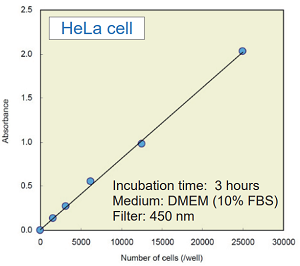
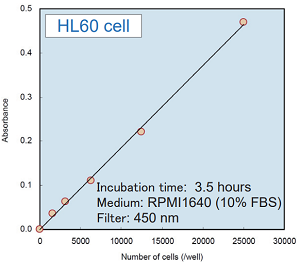
Experimental example : the number of cells vs. absorbance
Cell Viability Assay
- Collect cells and wash them with the medium. Prepare the cell suspension at the optimum concentration which is determined in a preliminary experiment.
- Add 100 μl of the cell supension to each well of a 96-well tissue culture plate.
- For adherent cells, please incubate the culture plate overnight to attach the cells to the plate.
- Add 10 μl of the medium containing test substance that adjusted to the desired concentration. Incubate the plate at 37°C for an appropriate time(e.g. 6, 12, 24, or 48 hours) in a CO2 incubator.
- In case the test substance has color or reducing activity, prepare “Blank” well which includes test substance, CCK-8, and medium without cells.
- Add 10 μl of the CCK-8 solution to each well of the plate.
- Be careful not to introduce bubbles to the wells, since they interfere with the O.D. reading.
- Incubate the plate at 37℃ for 1 - 4 hours in the CO2 incubator.
- Please refer to the determined optimum incubation time in preliminary experiment.
- Measure the absorbance at 450 - 490 nm by using a microplate reader.
Calculation of Cell Survival Rate
Calculate the average absorbance from each triplicate set of wells and subtract the absorbance of “Blank” from each absorbance of “Sample” and “Control” in the following equation.
-
Control : CCK-8, medium and cells in a well Blank : CCK-8 and medium without cells in a well Sample : CCK-8, medium, cells and test substances in a well
LDH Assay for cell death detection LDH Assay
There are two methods: homogeneous assay and non-homogeneous assay, please choose the assay method according to your experiments.
-
Homogeneous assay
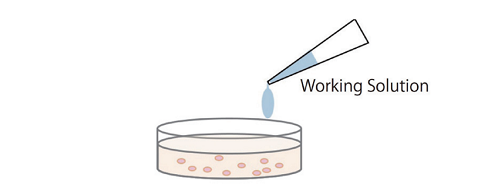
Simple procedure: homogeneous assay only requires adding the Working Solution to each well in the presence of live and dead cells, there is no need to transfer cell culture supernatant to a new microplate for measurement.
-
Non-homogeneous assay
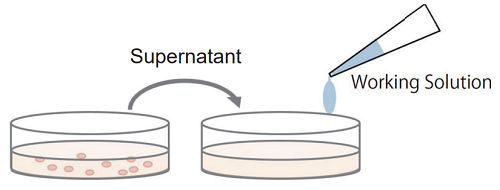
Multi-measuring use: since non-homogeneous assay uses the culture supernatant from each well for the measurement of LDH activity, the sample cells in each well can be applied to some other experiments such as cell viability assay (WST-8 and MTT) and cell staining (nuclear staining, immunostaining).
Preparation of Reagent
- To dissolve the powder, add 5 ml of Assay Buffer to the Dye Mixture vial. Close the cap and dissolve the contents completely.
- Add the whole volume of the mixture prepared in 1) to the Assay Buffer bottle.
- Store the Working Solution at 0-5℃ and protect it from light. It is stable for 6 months.
Homogeneous assay
Optimization of Cell Concentration
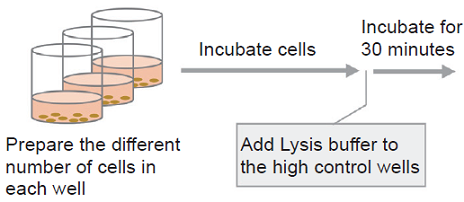
- Collect cells and wash them with the medium. Prepare 5×105 cells/ml cell suspension in the medium.
- Prepare 100 μl of 2-fold serially diluted cell suspension in a 96-well tissue culture plate.
- Refer the Serial Dilution Procedure.
- Incubate cell suspension at 37℃ for an appropriate time in a CO2 incubator.
- Add 10 μl of Lysis Buffer to each well of the high control.
- Incubate the plate at 37℃ for 30 minutes in the CO2 incubator.
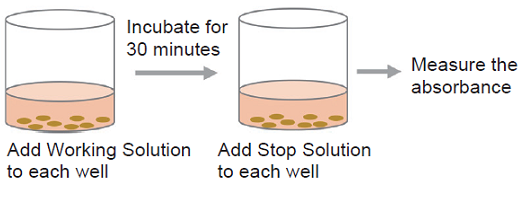
- Add 100 μl of Working Solution to each well. Protect the plate from light and incubate it at room temperature for 30 minutes.
- Add 50 μl of Stop Solution to each well.
- Measure the absorbance at 490 nm by using a microplate reader.
- Plot the data by setting cell concentrations on the x-axis and absorbances on the y-axis. The plot should have two linear lines, one representing High Control and another Low Control. Determine the optimum concentration of cells based on the followings.
Eliminate the concentration of cells that has absorbances higher than 2.0.
Choose the concentration of cells that has at least 0.2 difference in the absorbance between high control and low control.
- Plot the data by setting cell concentrations on the x-axis and absorbances on the y-axis. The plot should have two linear lines, one representing High Control and another Low Control. Determine the optimum concentration of cells based on the followings.
-
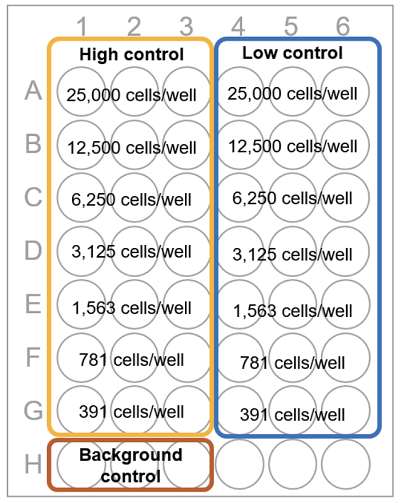
Fig. 1 Plate arrangement
-
Table 1 Amount of each solution (Homogeneous assay) Test substance High control Note: Low control Background control Medium - 50 μl 50 μl 100 μl Cell suspension 50 μl 50 μl 50 μl - Test substance in culture medium 50 μl - - - Lysis Buffer - 10 μl - - - The difference of total volume of test substance and high control does not affect the result.
Test substance : The released LDH activity from the cell when the test substance is added to the cell
High control : The total LDH activity in the cell (= Maximum LDH release)
Low control : The LDH activity released from the untreated cells to the medium (= Spontaneous LDH release)
Background control : The LDH activity in the medium*
- medium = culture medium
Cytotoxicity Assay
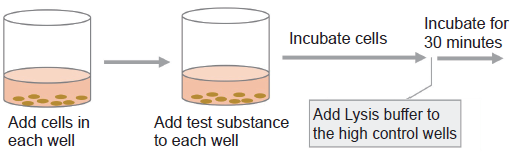
- Add 50 μl of cell suspension to each well of a flat-bottom 96-well tissue culture plate.
- Prepare the double concentration of cell suspension which is confirmed in the preliminary experiment.
For adherent cells: incubate the plate at 37℃ overnight in a CO2 incubator to allow the cells to adhere and then replace the medium with 50 μl of fresh medium.
- Prepare the double concentration of cell suspension which is confirmed in the preliminary experiment.
- Add 50 μl of medium containing test substance that adjusted to the desired concentration (Refer to Table 1).
- Incubate the plate at 37℃ for an appropriate time in a CO2 incubator.
- Add 10 μl of Lysis Buffer to each well of the high control. Incubate the plate at 37℃ for 30 minutes in a CO2 incubator.
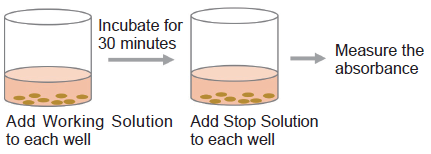
- Add 100 μl of Working Solution to each well. Protect the plate from light and incubate it at room temperature for 30 minutes.
- Add 50 μl of Stop Solution to each well.
- Measure the absorbance at 490 nm by using a microplate reader.
Calculation of Cytotoxicity
Calculate the average absorbance from each triplicate set of wells and subtract the absorbance of background from each absorbance of test substance, high control, and low control. Determine the percent cytotoxicity by the following equation.
-
A: Test substance
B: High control
C: Low control
Non-homogeneous assay
Optimization of Cell Concentration
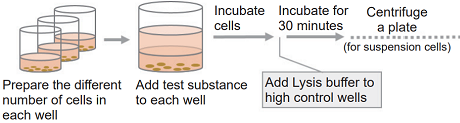
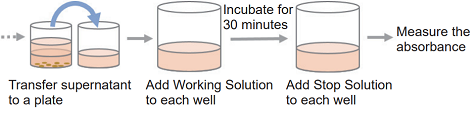
- Collect cells and wash them with the medium. Prepare 5×105 cells/ml cell suspension in the medium.
- Prepare 2-fold serially diluted cell suspension in a 96-well tissue culture plate.
- Refer the Serial Dilution Procedure.
- Add 100 μl of the cell suspension and 100 μl of the medium to each well.
- Incubate the plate at 37℃ for an appropriate time in a CO2 incubator.
- Add 20 μl of Lysis Buffer to each well of the high control.
- Incubate the plate at 37℃ for 30 minutes in the CO2 incubator.
- Centrifuge the plate at 250 × g for 2 minutes to precipitate the cells (for suspension cells).
- Transfer 100 μl of the supernatant from each well to a 96-well clear plate.
- Add 100 μl of Working Solution to each well. Protect the plate from light and incubate it at the room temperature for 30 minutes.
- Add 50 μl of Stop Solution to each well.
- Measure the absorbance at 490 nm by using a microplate reader.
- Plot the data by setting concentration of cells on the x-axis and absorbances on the y-axis. The plot should have two linear lines, one representing High Control and another Low Control. Determine the optimum concentration of cells based on the followings.
Eliminate the concentration of cells that has absorbances higher than 2.0.
Choose the concentrations of cells that has at least 0.2 difference in the absorbance between high control and low control.
- Plot the data by setting concentration of cells on the x-axis and absorbances on the y-axis. The plot should have two linear lines, one representing High Control and another Low Control. Determine the optimum concentration of cells based on the followings.
Cytotoxicity Assay
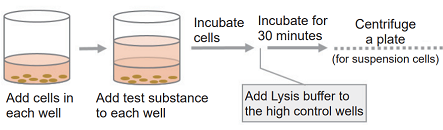
- Add 100 μl of the cell suspension to each well of a 96-well tissue culture plate.
- For adherent cells: incubate the plate at 37℃ overnight in a CO2 incubator to allow the cells to adhere and then replace the medium with 100 μl of fresh medium.
- Add 100 μl of the medium containing test substance that adjusted to the desired concentration (Refer to Table 2).
- Incubate the plate at 37℃ for an appropriate time in a CO2 incubator.
- Add 20 μl of Lysis Buffer to each well of the high control. Incubate the plate at 37℃ for 30 minutes in the CO2 incubator.
- Centrifuge the plate at 250 × g for 2 minutes to precipitate the cells (for suspension cells).
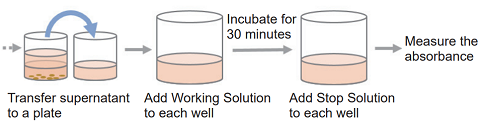
- Transfer 100 μl of the supernatant from each well to each well of a new 96-well clear plate.
- Add 100 μl of the Working Solution to each well. Protect the plate from light and incubate it at room temperature for 30 minutes.
- Add 50 μl of Stop Solution to each well.
- Measure the absorbance at 490 nm by using a microplate reader.
Calculation of Cytotoxicity
Calculate the average absorbance from each triplicate set of wells and subtract the absorbance of background from each absorbance of test substance, high control, and low control. Determine the percent cytotoxicity by the following equation.
-
A: Test substance
B: High control
C: Low control
-
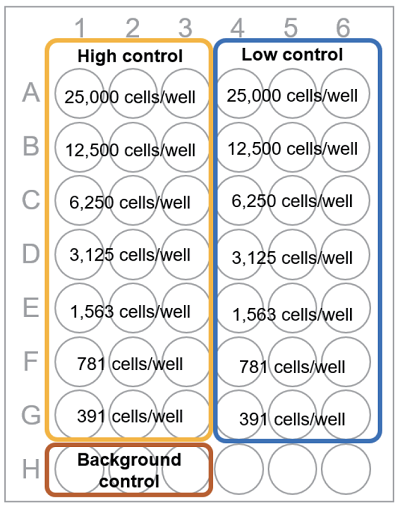
Fig. 2 Plate arrangement
-
Table 2 Amount of each solution (Non-homogeneous assay) Test substance High control Note: Low control Background contro Medium 20 μl 100 μl 120 μl 220 μl Cell suspension 100 μl 100 μl 100 μl - Test substance in culture medium 100 μl - - - Lysis Buffer - 20 μl - - Test substance : The released LDH activity from the cell when the test substance is added to the cell
High control : The total LDH activity in the cell (= Maximum LDH release)
Low control : The LDH activity released from the untreated cells to the medium (= Spontaneous LDH release)
Background control : The LDH activity in the medium*
- medium = culture medium
Experimental example for CCK-8 and LDH assay in same cells
Cell Viability and Cytotoxicity Assay
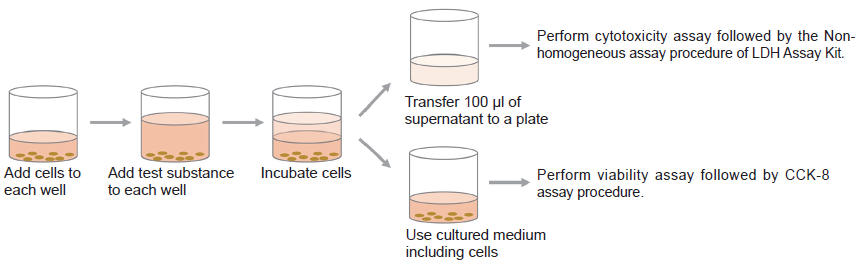
- Add 5000 cells/100 μl of HeLa cell to each well of a 96-well tissue culture plate.
- Incubate the plate at 37℃ overnight in a CO2 incubator.
- Add 100 μl of the medium containing test substance that adjusted to the desired concentration.
- Incubate the plate at 37℃ for one hour in the CO2 incubator.
- Settle the suitable incubation time for the experiment.
- Add 20 μl of Lysis Buffer to each well of the high control at 30 minutes before the end of incubation time.
LDH assay using supernatant (under the procedure of Non-homogeneous assay)
- Transfer 100 μl of the supernatant to a new 96-well clear plate.
- Add 100 μl of the Working Solution to each well.
- Protect the plate from light and incubate it at room temperature for 30 minutes.
- Add 50 μl of Stop Solution to each well.
- Measure the absorbance at 490 nm by using a microplate reader
CCK-8 assay using cultured medium with cells
- Add 10 μl of CCK-8 to each well where cells have been cultured.
- Incubate the plate at 37℃ for 3 hours in the CO2 incubator.
- Settle the suitable colorimetric reaction time for the experiment.
- Measure the absorbance at 450 nm by using a microplate reader.
Result of experimental example
-
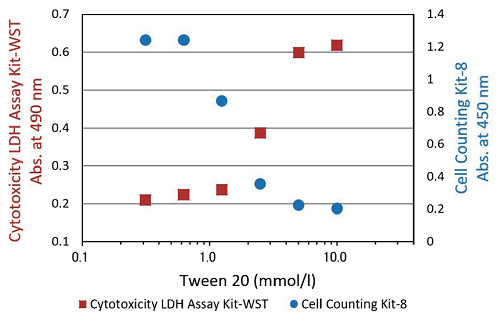
Fig.3 Cytotoxicity assay of Tween 20
-
Cell : HeLa cell Medium : MEM with 10% FBS Test substance : Tween 20 Drug exposure time : 1 hour
Serial Dilution Procedure
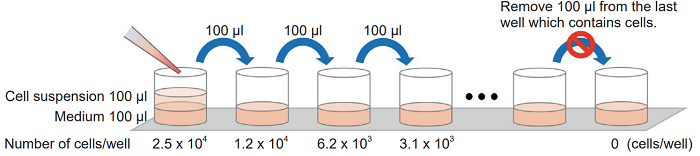
Add 100 μl of medium to each well of a 96-well tissue culture plate. Then, add the cell suspension (5 x 105 cells/ml) to the first well and mix by pipetting. This well contains the maximum number of cells (2.5 x 104 cells/well). Transfer 100 μl from the first well to the next well, and mix by pipetting. Repeat this procedure.
Procedure for serial dilution
Frequently Asked Questions / Reference
CK17: Viability/Cytotoxicity Multiplex Assay Kit
Revised Aug., 24, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.