General Information
Biofilms are biological aggregates held together by extracellular polysaccharides. They form at the solid–liquid interface in many environments where water is present. Bacterial contamination of medical devices, caries and periodontal diseases, food poisoning, metal corrosion, and drainage pipe contamination are examples of problems that are attributable to biofilms. Therefore, research into materials that have anti-biofilm activities is a growing area.
The Biofilm TestPiece Assay Kit measures the amount of biofilm formation on test pieces by crystal violet staining. By using the TestPiece Holder, multiple samples can easily be washed and measured at once without peeling the biofilm off for each process.
Kit Contents
| 24 tests | |
| TestPiece Holder | × 1 |
| Crystal Violet Solution | 50 ml × 1 |
Storage Condition
Store in a cool and dark place.
Required Equipment and Materials
-
- Test pieces (size: 20 × 10 × 1–3 mm (Length × Width × Thickness))
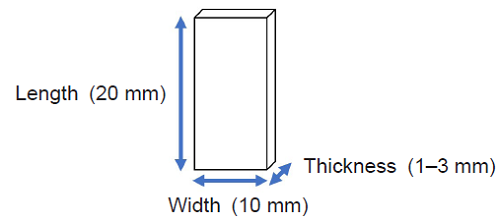
- Test pieces (size: 20 × 10 × 1–3 mm (Length × Width × Thickness))
-
- Falcon® 24-well plates (Dojindo cat# B608)
- Microplate reader (around 590 nm filter)
- Incubator
- 1000–5000 μl pipette
- Ethanol
- Sterile physiological saline solution
Precaution
- The conditions for biofilm formation vary by species of bacteria. Please refer to Table 1 for optimized culture conditions for biofilm formation.
- It is recommended not to reuse the TestPiece Holder to avoid contamination by unwanted bacteria and not to sterilize by heat because of its deformation.
- Falcon® brand 24-well plates are confirmed to be suitable for the TestPiece Holder (available from Dojindo, cat#B608).
-
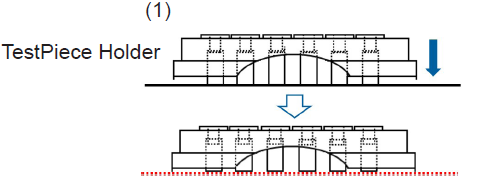
- The TestPiece Holder has a proper orientation. Please make sure the protrusion of the TestPiece Holder is above the "FALCON" carved seal as shown in Figure 1.
-

Figure 1 How to cover TestPiece Holder and 24-well plate
General Protocol
Measuring the amount of biofilm formation
-
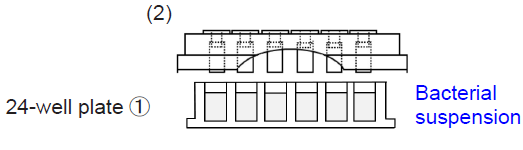
1. Fix test pieces to the TestPiece Holder.
- Please handle test pieces with care to avoid scratch and contamination when fixing test pieces with tweezers, etc.
- Insert test pieces into the clip of the holder, then press the Holder against a flat, clean surface to align the pieces.
- A video how to fix test pieces is available at Dojindo's website.
-
2. Add 1.8 ml of bacterial suspension to each well of 24-well plate①. Place the TestPiece Holder onto 24-well plate① and then incubate the plate at optimum temperature to form biofilms on the test pieces.
- The condition for biofilm vary by species of bacteria (Table 1).
-
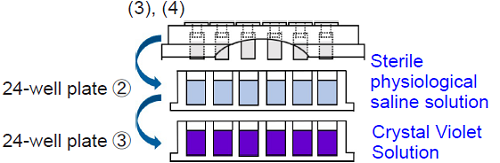
3. Add 2.0 ml of sterile physiological saline solution to each well of 24-well plate②. Add 2.0 ml of Crystal Violet Solution to each well of 24-well plate③.
4. Wash the TestPiece Holder from step 2 by soaking and shaking vertically and horizontally in 24-well plate② for 30 seconds. Then, place the TestPiece Holder onto 24-well plate③. Incubate the plate at room temperature for 30 minutes.
- Please soak the test pieces in saline gently and avoid vigorous shaking to prevent peeling off the biofilm and shifting the test pieces' position.
-
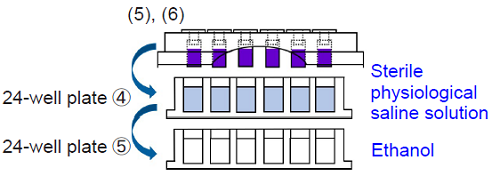
5. Add 2.0 ml of sterile physiological saline solution to each well of 24-well plate④. Add 2.0 ml of ethanol to each well of 24-well plate⑤.
6. Wash the TestPiece Holder from step 4 by soaking and shaking vertically and horizontally in 24-well plate④ for 30 seconds. Then, place the TestPiece Holder onto 24-well plate⑤. Incubate the plate at room temperature for 30 minutes.
- Please soak the test pieces in saline gently and avoid vigorous shaking to prevent peeling off the biofilm and shifting the test pieces' position.
- The extraction efficiency of crystal violet may vary depending on the biofilm formation. To optimize the efficiency, please investigate a kind of extracting solution other than ethanol.
-
7. Remove the TestPiece Holder and measure the absorbance at around 590 nm using a microplate reader.
- If the absorbance is too high to measure, please dilute the solution in the 24-well plate.
Experimental Example 1
Measurement of Staphylococcus aureus biofilm formation on polystyrene
- S. aureus biofilm formation requires two periods of incubation for 24 h (see Table 1).
- The test pieces (20 × 10 × 2 mm (Length × Width × Thickness)) were fixed to the TestPiece Holder.
- Bacterial suspension was prepared at approximately 107 colony-forming units (CFU)/ml with Mueller-Hinton broth (MHB), and 1.8 ml of bacterial suspension was added o each well of 24-well plate ①.
- The TestPiece Holder was then placed onto 24-well plate①, and the plate was incubated at 37 °C for 24 hours.
- 24-well plate ② was prepared by placing 1.8 ml of MHB in each well. The TestPiece Holder from step 3 was placed onto 24-well plate ②, and the plate was incubated at 37 ℃ for 24 hours.
- Sterile physiological saline solution (2.0 ml) was added to each well of 24-well plate ③. Crystal violet solution (2.0 ml) was added to each well of 24-well plate ④.
- The TestPiece Holder from step 4 was washed by soaking and shaking lightly in 24-well plate③ for 30 seconds. The TestPiece Holder was then placed onto 24-well plate ④, and the plate was incubated at room temperature for 30 minutes.
- Sterile physiological saline solution (2.0 ml) was added to each well of 24-well plate ⑤. Ethanol (2.0 ml) was added to each well of 24-well plate ⑥.
- The TestPiece Holder from step 6 was washed by soaking and shaking lightly in 24-well plate⑤ for 30 seconds. The TestPiece Holder was then placed onto 24-well plate ⑥, and the plate was incubated at room temperature for 30 minutes.
- The TestPiece Holder was removed, diluted 4-fold with ethanol, and the absorbance of each well at 595 nm was measured.
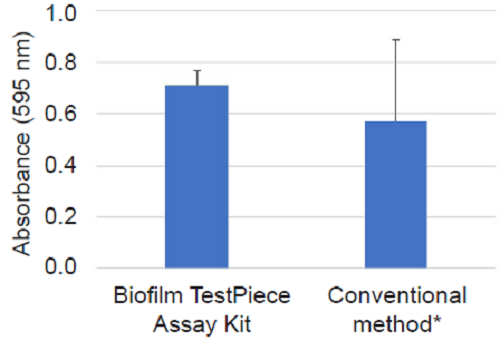
Figure 2 Measurement of S. aureus biofilm formation on polystyrene
The Biofilm TestPiece Assay Kit gives less dispersion than the conventional method.
- Conventional method for evaluating biofilm formation on a test piece left in the bottom of a container such as a petri dish or beaker.
Experimental Example 2
Measurement of Staphylococcus aureus biofilm formation on different materials
The protocol was the same as the experimental example 1.
-
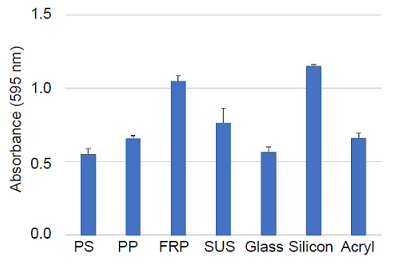
Figure 3 Amount of biofilm formation on each material
-
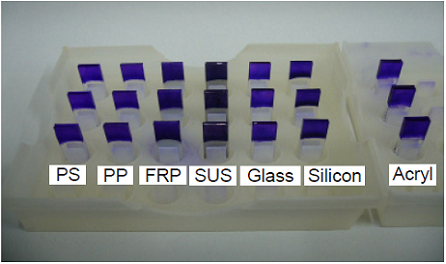
Figure 4 Image of Staphylococcus aureus
biofilm formation on each material
| Bacteria | Bacteria dilution medium |
Bacterial concentration (CFU/ml) |
Incubation time (h) |
| Pseudomonas aeruginosa | Brain Heart Infusion | 107 | 5–6 |
| Staphylococcus aureus | Mueller-Hinston Broth | 107 | 48* |
| Escherichia coli | Brain Heart Infusion | 107 | 72* |
- The medium is changed every 24 hours for culturing.
This product was developed through joint research within the Biotechnology and Food Research Institute, Fukuoka Industrial Technology Center.
------------------------------------------------------------------------------------------------------------------------------------------------------------
Falcon is a registered trademark of Corning Incorporated, Life Sciences.
Product Number: 353047
Falcon® 24-well Clear Flat Bottom TC-Treated Multiwell Cell Culture Plate, with Lid, Sterile
------------------------------------------------------------------------------------------------------------------------------------------------------------
Frequently Asked Questions / Reference
B606: Biofilm TestPiece Assay Kit
Revised Jun., 01, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.