General Information
Various methods are available to immobilize proteins on a gold surface through self-assembled monolayers (SAMs) to create biosensors used in a QCM (Quartz Crystal Microbalance) or a SPR (Surface Plasmon Resonance) such as to make a covalent bond with proteins through an activated carboxylic group on the SAM surface, to fix Histidine-tagged protein (His-tag protein) through a Ni-NTA chelate on the surface, and to fix biotinylated protein through a streptavidin molecule on SAM surfaces. Biotin-avidin method is the most popular system to immobilize protein on a sensor due to the quick and very stable binding. Biotin-SAM Formation Reagent is specifically designed to prepare avidin-coated biosensors using streptavidin or NeutrAvidin. The biotinylated-surface prepared with this reagent can hold more streptavidin than conventional biotin-SAM surfaces. Additionally, the streptavidin surfaces prepared with this reagent can minimize non-specific protein adsorption.
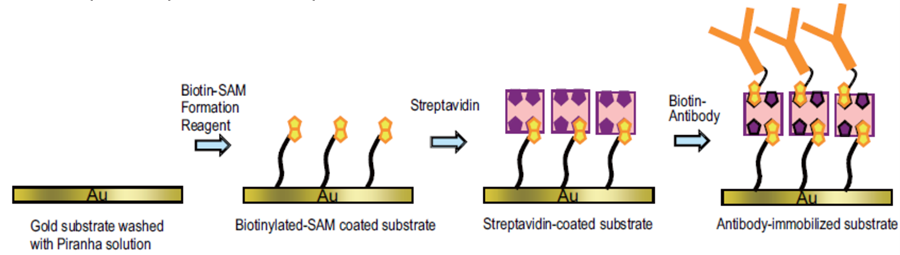
Fig.1 Preparation of a biosensor using Biotin-SAM Formation Reagent and streptavidin.
Contents
| Biotin-SAM Formation Reagent | 1 μmol x 3 |
Storage
Store at 0-5°C.
Biotin-SAM General Protocol
Preparation of a Biotin-SAM surface on a gold substrate
- Add 1 ml ethanol to a tube, and pipette to dissolve the reagent to prepare 1 mmol/l Biotin-SAM solution.*1 Then, dilute the solution 10-fold with ethanol for Step 2.
- Clean a gold surface of a substrate with Piranha solution *2 prior to preparing Biotin-SAM. Immerse the substrate in the reagent solution prepared at Step 1 at room temperature and leave it for 1 hour.
- Wash the substrate several times with ethanol and purified water sequentially.*3
- If the reagent does not dissolve by pipetting, use an ultrasonic bath or warm the tube around 40℃. Use freshly prepared reagent solution at Step 2.
- Mix 3 volumes of sulfuric acid and one volume of hydrogen peroxide solution to prepare Piranha solution. Since Piranha solution is severely caustic, handle with great care.
- Store the SAM-coated substrate under nitrogen gas in a tightly sealed glass container at 0-5℃.
Experimental Example
Monitoring of protein binding processes on the biotin-SAM surface with QCM.
- The Biotin-SAM-coated substrate was attached to a cell of a QCM instrument and set the cell on the instrument according to the manufacturer's manual.
- Four hundred fifty μl PBS was added to the cell. After the stabilization of the frequency, 20 μl streptavidin solution (10 mg streptavidin dissolved with 1 ml PBS) was applied.A)
- After the stabilization of the frequency, frequency was monitored to evaluate non-specific binding of BSA (bovine serum albumin) and FBS (fatal bovine serum) by adding 8 μl BSA solution (10 mg BSA dissolved with 1 ml PBS) and then by adding 8 μl FBS solution (10 mg FBS dissolved with 1 ml PBS).B)
- The solution was removed and the cell was washed with PBS several times.
- Four hundred fifty μl PBS was added to the cell.
- After the stabilization of the frequency, 8 μl Biotin-BSA solution (12 mg Biotin-BSA dissolved with 1 ml PBS) was applied. C)
- The frequency was measured to monitor an immobilization of Biotin-BSA to the streptavidin-coated substrate.
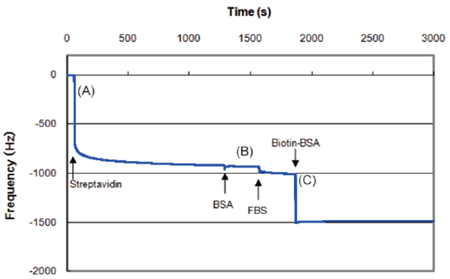
Fig. 2 Frequency monitoring during protein binding processes by QCM instrument.
-
Notes)
Data was prepared by AFFINIXQNμ (Initium).A) A large decrease in frequency was observed due to the streptavidin binding on the surface.
B) Very slight frequency change was caused by non-specific FBS binding.
C) A large and immediate change in frequency was observed by Biotin-BSA binding on the surface.
Streptavidin surface prepared with Biotin-SAM Formation Reagent minimize the unspecific binding of undesired protein such as BSA or FBS. Since the cell was washed at step 4. , the data at step 4. is left out.
Frequently Asked Questions / Reference
B564: Biotin-SAM Formation Reagent
Revised Dec., 11, 2023


 Hidden sections will not be printed.
Hidden sections will not be printed.

