| New Insights Reveal How Senescence Spreads and Enlarged Cells Are Sustained Aging is increasingly seen as a key factor behind many diseases, driving the search for ways to slow or prevent it. Recent work has shown that the protein HMGB1 can transmit senescence signals between cells and even to distant tissues, and that blocking this process supports tissue repair. Another study identified AP2A1 as a factor that keeps senescent cells enlarged, with its inhibition reversing aging traits. Together, these findings point to new possibilities for targeting the fundamental mechanisms of aging to improve health and longevity. |
||||||||||||||||||||||
| Reference | Related technique | |||||||||||||||||||||
| Propagation of senescent phenotypes by extracellular HMGB1 is dependent on its redox state (Metabolism, 2025) Summary: This study identifies redox-sensitive HMGB1 (ReHMGB1) as a novel driver of cellular senescence that spreads from one cell to its neighbors and through the bloodstream to distant organs. Blocking extracellular HMGB1 was shown to reduce systemic senescence and improve tissue regeneration, suggesting its potential as a therapeutic target for aging-related diseases. Highlighted technique: The study evaluated cellular senescence using SA β gal staining to identify senescent cells and EdU incorporation to assess proliferative arrest. Expression levels of p16 and p21 were also measured as molecular markers of cell cycle inhibition. |
 |
|||||||||||||||||||||
|
AP2A1 modulates cell states between senescence and rejuvenation (Cellular Signalling, 2025) Highlighted technique: The study combined SA β gal staining with quantitative analysis of cell size to directly link senescence associated β galactosidase activity to the pronounced hypertrophy of senescent cells. This morphological and biochemical readout was then applied to assess the effects of AP2A1 knockdown on SA β gal positivity and cellular enlargement. |
 |
|||||||||||||||||||||
Related Techniques (click to open/close)
|
||||||||||||||||||||||
Application Note (click to open/close)
|
||||||||||||||||||||||
|
|
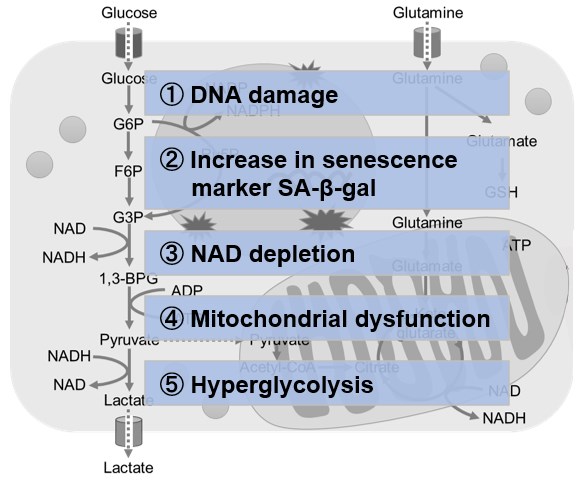
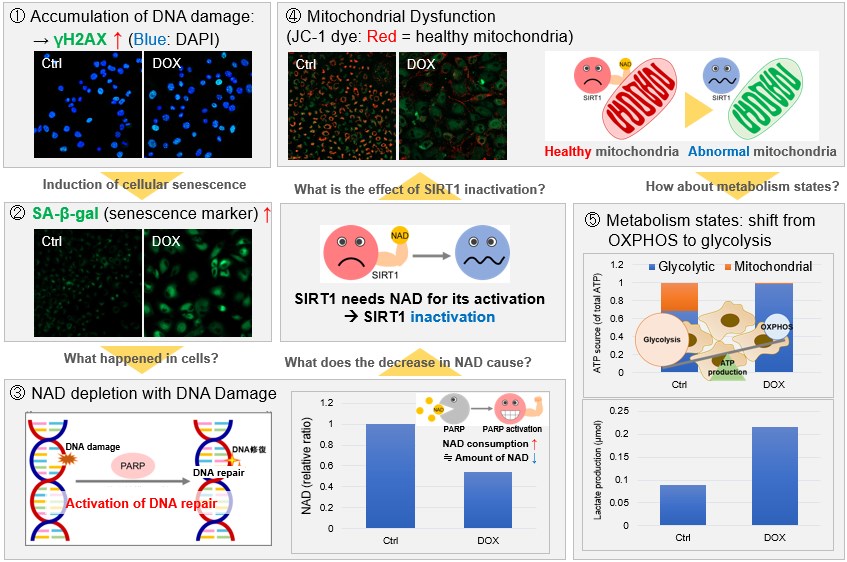
NAD(+) levels decline during the aging process, causing defects in nuclear and mitochondrial functions and resulting in many age-associated pathologies*. Here, we try to redemonstrate this phenomenon in the doxorubicin (DOX)-induced cellular senescence model with a comprehensive analysis of our products. *S. Imai, et al., Trends Cell Biol, 2014, 24, 464-471
|
 |
|