| Cellular senescence is recognized as an important contributor to cancer development through its effects on the tumor microenvironment, and also to age-related dysfunction in both the peripheral and central nervous systems. This ScienceNote introduces recent findings that highlight the tumor-promoting role of senescent fibroblasts in breast cancer, the contribution of senescent neurons to age-associated pain, and the accumulation of senescent neurons through abnormal cell cycle activity in neurodegenerative conditions. | ||||||||||||||||||
|
Senescent CAFs mediate immunosuppression and drive breast cancer progression (Cancer Discov., 2025) Highlighted technique: To examine how senescent CAF-derived ECM affects NK cell function, doxorubicin-treated mouse mammary fibroblasts were cultured for six days to deposit ECM in vitro. After cell removal, the ECM was used in NK cytotoxicity assays, with NK cells pre-incubated on the matrix before co-culture with labeled target cells. Related technique SA-βGal Detection(Cell) |
||||||||||||||||||
|
Aging and injury drive neuronal senescence in the dorsal root ganglia (Nature Neuroscience, 2025) Highlighted technique: This study assessed DRG neuron senescence using multiple markers. Aging-related senescence was confirmed by increased SA-β-gal activity, and injury-induced senescence was evaluated by examining the upregulation of p16, p21, and SASP factors including IL-6. Related technique SA-βGal Detection(Tissue) |
||||||||||||||||||
|
Neuronal cell cycle reentry events in the aging brain are more prevalent in neurodegeneration and lead to cellular senescence (PLoS Biol., 2024) Highlighted technique: To identify rare neurons that start dividing again, the researchers analyzed RNA data from individual cell nuclei collected from multiple human brain samples. They used a list of 350 genes related to the cell cycle and a scoring method to find excitatory neurons that are normally non-dividing but showed signs of abnormal cell cycle activity. Related technique Cell Cycle Assay |
||||||||||||||||||
Related Techniques (click to open/close)
|
||||||||||||||||||
Application Note I (click to open/close)
|
||||||||||||||||||
|
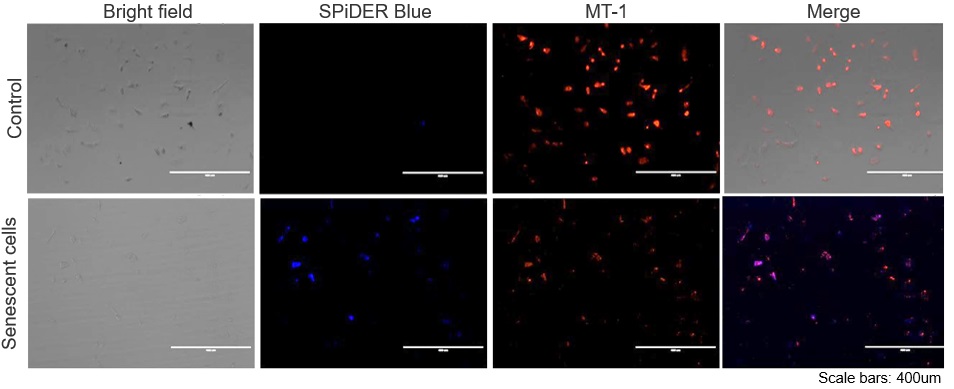
The senescent cell detection dye SPiDER Blue (SG07) and the mitochondrial membrane potential (MMP) dye MT-1 (MT13) were used to stain human microglial cells. Microscopy revealed that, compared to control cells, senescence-induced cells showed reduced MMP and increased SPiDER Blue fluorescence, reflecting elevated SA-β-Gal activity. *This data was kindly provided by Dr. Supriya D. Mahajan, Department of Medicine, Jacobs School of Medicine & Biomedical Sciences. 1. Seed human microglia cells into a dish and incubate in an incubator set at 37 ℃ and equilibrated with 95% air and 5% CO2. |
Application Note II (click to open/close)
> Increased Senescence in Aged Adipose Tissue
|
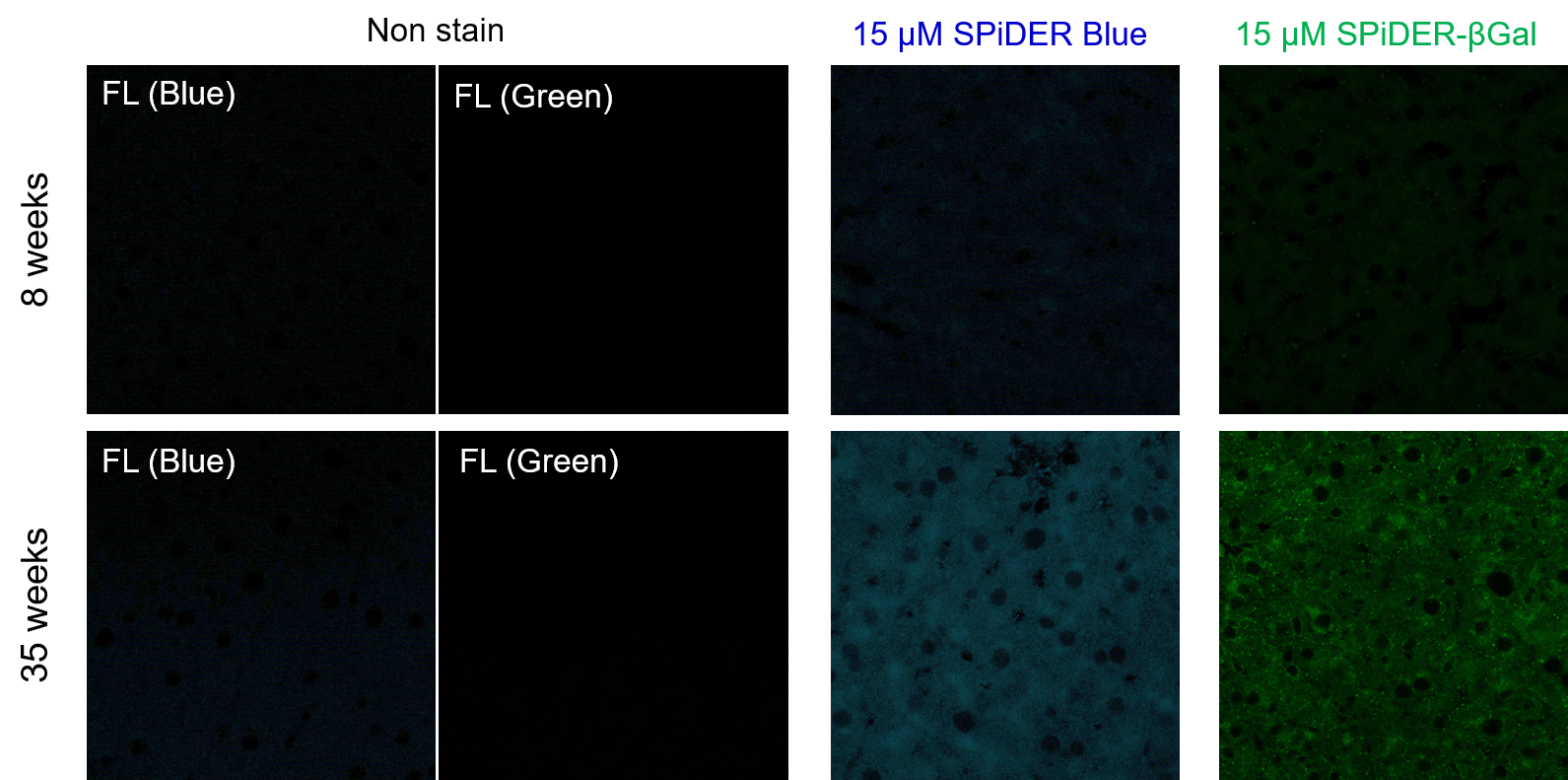
Frozen liver adipose tissue sections from 8-week-old and 35-week-old mice were stained with senescence detection probes SPiDER Blue (SG07) and SPiDER-βGal (SG02). Confocal microscopy revealed a marked increase in fluorescence intensity only in the 35-week-old samples, indicating an age-associated accumulation of senescent cells in older tissue. 1. 8-week-old and 35-week-old mouse liver adipose tissue (frozen sections) samples were prepared on glass slides. [Detection conditions] |