|
Some research on Alzheimer's disease (AD) shows that lysosomal dysfunction is observed in the early stages of AD and affects the progression of AD. Here are some of the studies that show one of the mechanisms of lysosomal dysfunction in AD and ideas for therapy.
Lysosomes play a critical role in the removal and degradation of cellular waste, including the degradation of proteins and damaged organelles by autophagy. Lysosomal dysfunction is commonly observed in Alzheimer's disease (AD), contributing to the accumulation of amyloid beta plaques and tau tangles that are hallmarks of the disease. Impaired lysosomal activity disrupts cellular homeostasis, leading to the accumulation of toxic proteins and organelle damage, further exacerbating neurodegeneration. Restoring lysosomal function has emerged as a potential therapeutic target for slowing or ameliorating the progression of Alzheimer's disease.
|
|
-
Accumulation of APP C-terminal fragments causes endolysosomal dysfunction through the dysregulation of late endosome to lysosome-ER contact sites
Click here for the original article:Marine Bretou, et. al.,Developmental Cell, 2024.
Point of Interest
- Endolysosomal collapse in Alzheimer's disease (AD), initiated by decreased lysosomal calcium and increased cholesterol, is triggered by γ-secretase inhibition, with APP depletion rescuing these dysfunctions.
- APP C-terminal fragments (CTFs) are localized to late endosome-lysosome-ER contacts, and excess APP CTFs cause lysosomal Ca2+ deficits, leading to cholesterol accumulation at endosome-lysosome-ER contacts.
- Failure to maintain balanced APP-CTF levels perpetuates signaling, causing lysosomal dysfunction and contributing to early AD pathology.
-
Urolithin A improves Alzheimer's disease cognition and restores mitophagy and lysosomal functions
Click here for the original article:Yujun Hou, et. al., Alzheimers Dement., 2024.
Point of Interest
- Long-term treatment with urolithin A (UA) improves learning, memory and olfactory function in Alzheimer's disease mice.
- UA reduces amyloid beta and tau pathology and improves mitophagy by restoring lysosomal function through cathepsin Z regulation.
- UA shows therapeutic potential for Alzheimer's disease by improving lysosomal function and modulating immune responses and Alzheimer's disease-related pathways.
-
Rescue of ApoE4-related lysosomal autophagic failure in Alzheimer’s disease by targeted small molecules
Click here for the original article:Meenakshisundaram Balasubramaniam, et. al., Communications Biology, 2024.
Point of Interest
- The APOE ε4 allele increases the risk of Alzheimer's disease by impairing autophagy by blocking the transcription of autophagy genes.
- Small molecules have been identified that bind ApoE4 and restore autophagy gene transcription, thereby reducing amyloid aggregation in models.
- These findings suggest that ApoE4-targeted drugs could be used to rescue lysosomal autophagy in neurodegenerative diseases such as Alzheimer's.
|
|
Related Techniques
|
- Lysosomal function
- Lysosomal Acidic pH Detection Kit -Green/Red and Green/Deep Red
|
- First-time autophagy research
- Autophagic Flux Assay Kit
|
- Autophagy detection
- DAPGreen/ DAPRed (Autophagosome detection), DALGreen (Autolysosome detection)
|
- Mitophagy detection
- Mitophagy Detection Kit
|
- Mitochondrial membrane potential detection
- JC-1MitoMPDetection Kit,MT-1MitoMPDetection Kit
|
- Glycolysis/Oxidative phosphorylation Assay
- Glycolysis/OXPHOS Assay Kit, Extracellular OCR Plate Assay Kit
|
- Oxygen consumption rate assay
- Extracellular OCR Plate Assay Kit
|
- Intracellular calcium ion detection
- Fura 2-AM
|
|
|
- Cell Proliferation / Cytotoxicity Assay
- Cell Counting Kit-8, Cytotoxicity LDH Assay Kit-WST
|
|
Related Applications
|
Analysis of autophagic flux without transfection
DALGreen and DAPRed labeled HeLa cells were used to evaluate changes in autophagic flux induced by the lysosomal acidification inhibitor bafilomycin A1 (Baf. A1). Compared to starvation conditions, the fluorescence signals of DALGreen were decreased under inhibited conditions of autolysosome formation by the addition of Baf. A1. In contrast, the fluorescence signals of DAPRed were increased under the same conditions, indicating that Baf. A1 led to the accumulation of autophagosome.
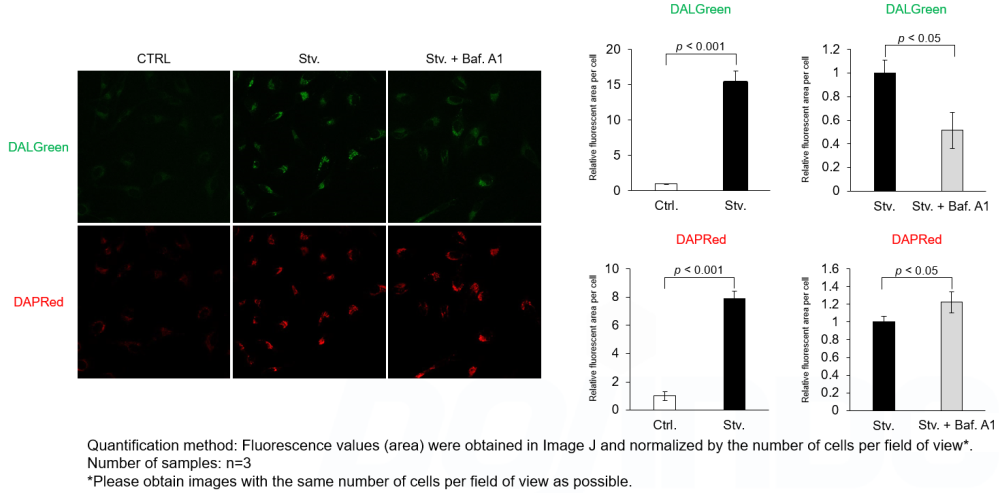
Experimental Conditions
CTRL: Normal condition, Stv.: Induction of autophagy, Stv. + Baf. A1: Inhibition of autolysosome formation
DALGreen filter set: 488 nm (Ex), 490–550 nm (Em)
DAPRed filter set: 561 nm (Ex), 565–700 nm (Em)
Products in Use
Autophagic Flux Assay Kit
|
















