|
Lysosomal dysfunction is increasingly recognized as a critical factor in the development and progression of several neurological diseases. In neurodegenerative diseases such as Parkinson's and Alzheimer's, impaired lysosomal function leads to the accumulation of misfolded proteins and neuronal toxicity, contributing to cell death and disease progression. Although rare, lysosomal storage diseases often have significant neurological manifestations due to the accumulation of undigested substances in neurons, impairing their function and survival. Therefore, understanding and targeting lysosomal pathways is emerging as a promising therapeutic approach to treat and potentially prevent a range of neurological disorders.
|
-
Lysosomes mediate the mitochondrial UPR via mTORC1-dependent ATF4 phosphorylation
Click here for the original article: Terytty Yang Li, et. al., Cell Discovery, 2023.
Point of Interest
- Mitochondrial stress activates mTORC1 through v-ATPase-mediated lysosomal stimulation, leading to phosphorylation of ATF4.
- This phosphorylation of ATF4 triggers the mitochondrial unfolded protein response (UPRmt).
- Interfering with the ability of mTORC1 to phosphorylate ATF4 inhibits the UPRmt.
- The phosphorylation of ATF4 prevents ROS-induced cell death under mitochondrial stress.
-
ARL8B mediates lipid droplet contact and delivery to lysosomes for lipid remobilization
Click here for the original article: Dilip Menon, et. al., Cell Reports, 2023.
Point of Interest
- ARL8B enhances the lysosomal lipolysis of triacylglycerol stored in lipid droplets (LDs).
- ARL8B in its GDP-bound state associates with LDs, whereas ARL8B-GTP is predominantly associated with lysosomes.
- ARL8B facilitates the contact between LDs and lysosomes, with both its GDP- and GTP-bound states forming a complex.
- The ARL8B-mediated lysosomal lipolysis of LDs represents a pathway for the turnover of neutral lipids in macrophages.
-
Nutrient-regulated control of lysosome function by signaling lipid conversion
Click here for the original article: Michael Ebner, et. al., Cell, 2023.
Point of Interest
- Starvation enhances lysosomal catabolism through the localized synthesis of PI(4)P on the lysosomal membrane.
- Cells possess diverse lysosome populations, distinguished by the presence of either PI(3)P or PI(4)P.
- A lipid switch, regulated by nutrient availability, facilitates the interconversion between these distinct lysosome populations.
- mTORC1 signaling and the synthesis of lysosomal PI(4)P are mutually inhibitory, each suppressing the other's activity.
|
|
Related Techniques
|
- Lysosomal function
- Lysosomal Acidic pH Detection Kit-Green/Red and Green/Deep Red
|
- Autophagy detection
- DAPGreen / DAPRed (Autophagosome detection), DALGreen (Autolysosome detection)
|
- Endocytosis detection
- ECGreen-Endocytosis Detection
|
- Phagocytosis detection
- AcidSensor Labeling Kit – Endocytic Internalization Assay and -Cellstain- Calcein-AM solution
|
- Mitophagy Detection
- Mitophagy Detection Kit and Mtphagy Dye
|
- Mitochondrial superoxide detection
- MitoBright ROS Deep Red - Mitochondrial Superoxide Detection
|
- Oxygen consumption rate assay
- Extracellular OCR Plate Assay Kit
|
- Antibody/Protein labeling - fast and high recovery
- Fluorescein, Biotin, and Peroxidase Labeling Kit - NH2
|
|
Related Applications
|
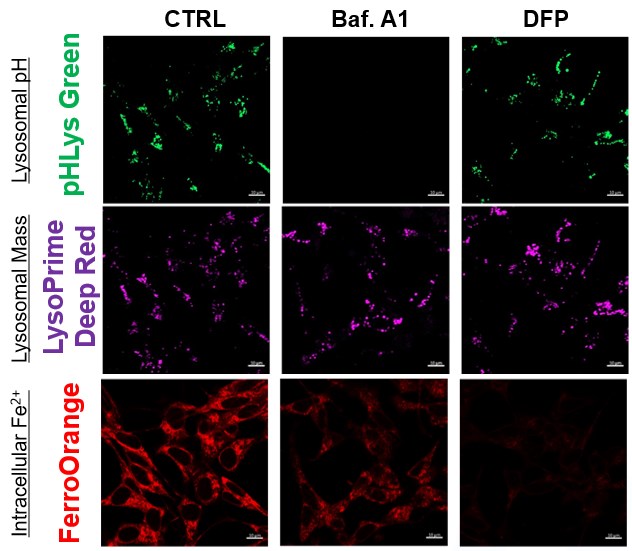
The simultaneous detection of lysosomal function with Mitochondrial ROS and intracellular Fe2+
Lysosomal Function and Iron Homeostasis
-
Recent reports suggest that lysosomal neutralization can result in iron depletion, consequently leading to the disruption of cell viability. To verify this, HeLa cells were labeled with FerroOrange for Fe2+ detection, and the lysosomal mass and pH were separately detected with LysoPrime DeepRed and pHLys Green (a product currently under development). Co-staining with FerroOrange and Lysosomal dyes demonstrated that Bafilomycin A1 (Baf. A1), an inhibitor of lysosomal acidification, causes iron depletion consistent with the findings reported in the article. Interestingly, the iron chelator, Deferiprone (DFP), did not impact lysosomal pH, suggesting that lysosomal function plays a key role in managing iron homeostasis.
Reference: Ross A Weber, et. al., Mol Cell (2020)
Products in Use
- FerroOrange
- pHLys Green*
- LysoPrime Deep Red
*pHLys Green will be available in July 2023 as the "Lysosomal Acidic pH Detection Kit-Green/Deep Red". If you would like to receive the promotional informaiton, please click here and write "New Product Information" in the inquiry box.
|
|
Lysosomal Function and Mitochondrial ROS
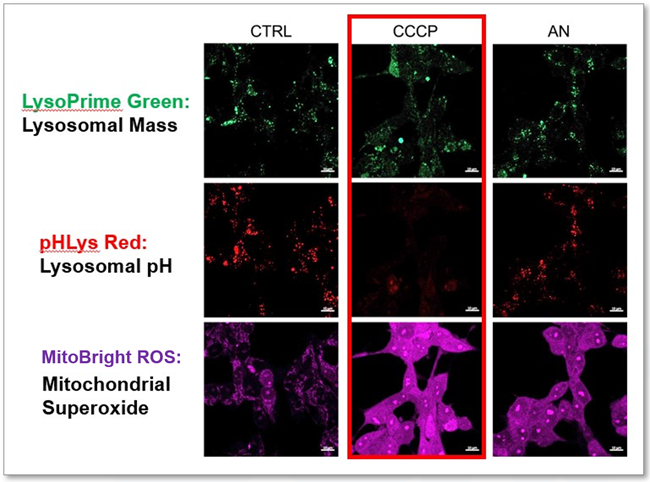
-
CCCP and Antimycin are recognized inducers of mitochondrial ROS, linked to the loss of mitochondrial membrane potential. Recent studies have shown that CCCP induces not only mitochondrial ROS but also lysosomal dysfunction. To observe mitochondrial ROS, HeLa cells were labeled with MitoBright ROS Deep Red for Mitochondrial Superoxide Detection, and the lysosomal mass and pH were independently detected with LysoPrime Green and pHLys Red. Co-staining with MItoBright ROS and Lysosomal dyes revealed that CCCP, unlike Antimycin, triggers concurrent lysosomal neutralization and mitochondrial ROS induction.
Reference: Benjamin S Padman, et. al., Autophagy (2013)
Products in Use
- LysoPrime Green
- pHLys Red
- Lysosomal Acidic pH Detection Kit
- MitoBright ROS Deep Red - Mitochondrial Superoxide Detection
|
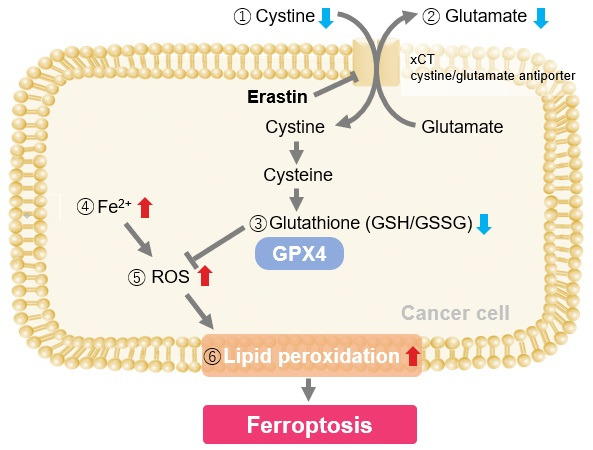
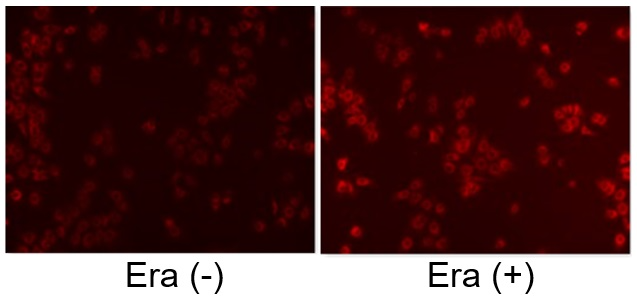
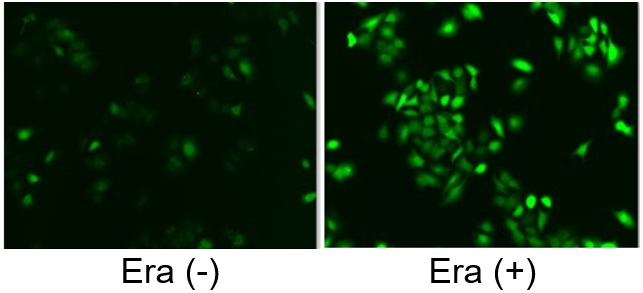
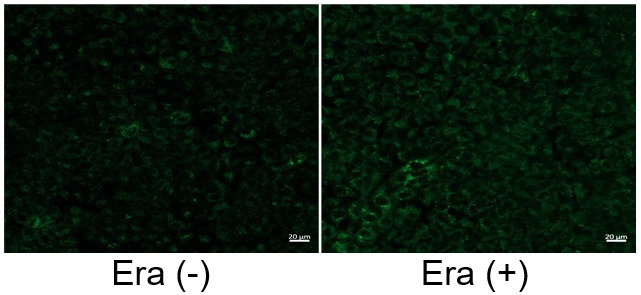
Induction of Ferroptosis by Erastin
Erastin is a known inducer of ferroptosis. By inhibiting the cystine transporter (xCT), erastin inhibits the uptake of cystine. Cystine is the raw material for GSH. Therefore, Erastin ultimately decreases the amount of GSH. Decreased GSH then results in lipid peroxide accumulation and induction of ferroptosis.
The following experimental examples show changes in each aforementioned index as a consequence of erastin stimulation. Measurements are made using Dojindo reagents.
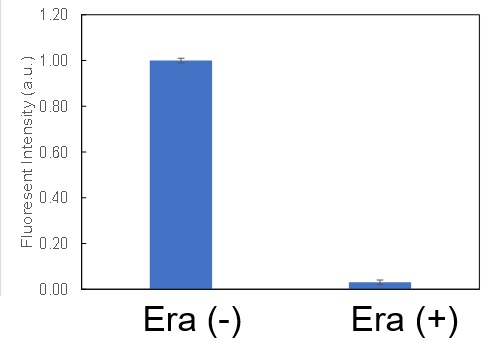
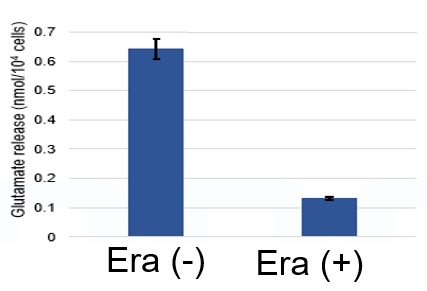
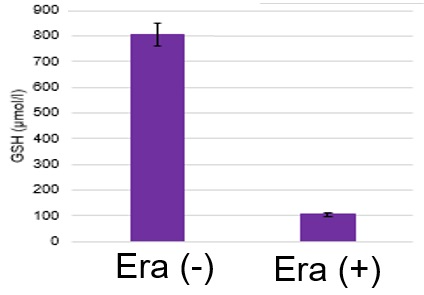
Using erastin-treated A549 cells, we measured intracellular Fe2+, ROS, lipid peroxide, glutathione, glutamate release into the extracellular space, and cystine uptake. As a result, inhibition of xCT by elastin was observed and also the release of glutamate and uptake of cystine were decreased. Furthermore, elastin treatment decreased intracellular glutathione while it increased intracellular Fe2+ , ROS, and lipid peroxides.
|