| [Aug. 20, 2024] | Previous Science Note |
|
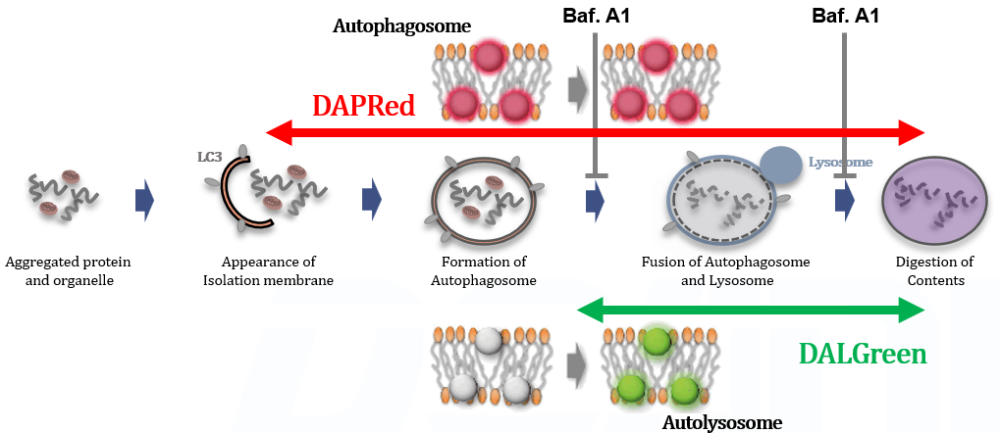
Cell death and the dysfunction of autophagy and lysosomes are closely linked in maintaining cellular health. When autophagy is impaired, cells cannot effectively degrade and recycle damaged components, leading to the accumulation of toxic materials and cellular stress. Lysosomal dysfunction exacerbates this problem, as these organelles are essential for the degradation of autophagosomal contents. The resulting cellular damage and stress can trigger cell death pathways that contribute to various diseases, including neurodegenerative disorders and cancer. |
|
|
Related Technique in This Topic |
|
|
|
|
|
|
|
|
|
Related Applications |
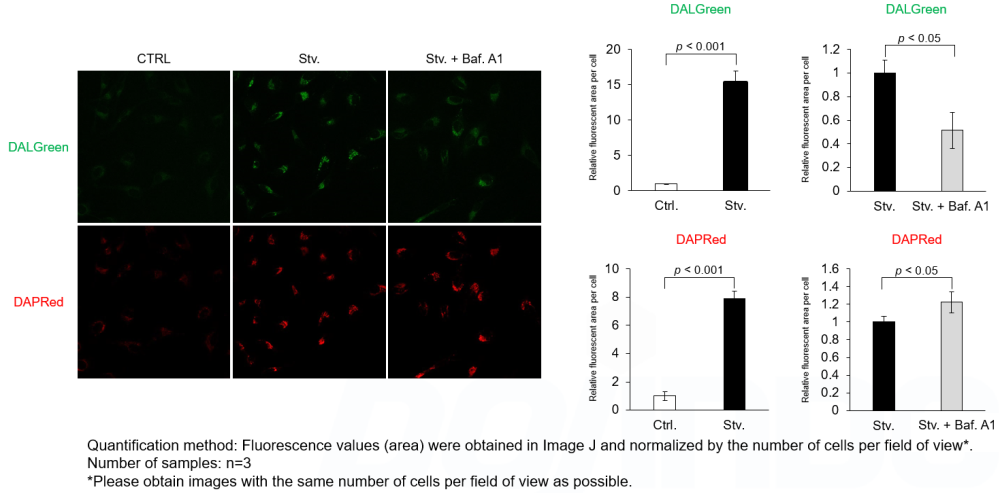
Analysis of autophagic flux without transfection
DALGreen and DAPRed labeled HeLa cells were used to evaluate changes in autophagic flux induced by the lysosomal acidification inhibitor bafilomycin A1 (Baf. A1). Compared to starvation conditions, the fluorescence signals of DALGreen were decreased under inhibited conditions of autolysosome formation by the addition of Baf. A1. In contrast, the fluorescence signals of DAPRed were increased under the same conditions, indicating that Baf. A1 led to the accumulation of autophagosome. Experimental Data
Experimental Conditions Procedure 1. HeLa cells were seeded (1.0 x 104 cells/well) on a μ-slide 8 well plate (ibidi) and cultured overnight at 37°C in an incubator equilibrated with 95% air and 5% CO2. 2. After washing twice with MEM containing 10% fetal bovine serum, 200 μl of DALGreen/DAPRed working solution (DALGreen: 1 µmol/l, DAPRed: 0.2 µmol/l) and the cells were incubated at 37°C for 30 minutes. 3. The supernatant was discarded, and the cells were washed twice with MEM containing 10% fetal bovine serum. 4. Samples were prepared under the following conditions. 5. The stained cells were observed under a confocal fluorescence microscope. Products in Use |