|
Scientists have discovered that protein aggregates and components of the autophagy machinery accumulate in developmental radial glia-like neural stem cells (NSCs) as they enter quiescence. They also uncover an important role of autophagy in the transition of developmental NSCs to their quiescent adult form by modulating autophagy activity in vivo.
|
|
Autophagy drives the conversion of developmental neural stem cells to the adult quiescent state
Click here for the original article: Isabel Calatayud-Baselga, et. al., Nature Communications, 2023.
Point of Interest
- Protein aggregates and autophagy components accumulate in radial glia-like NSCs during quiescence.
- Blocking autophagy hinders the establishment and maintenance of this quiescent state.
- Enhancing autophagy via AMPK/ULK1 activation promotes NSC quiescence independent of BMP signaling.
- Disruption of autophagy in radial glia-like NSCs affects initial and sustained quiescence during NSC niche formation in the hippocampal dentate gyrus.
|
|
Related Technique in This Topic
|
- Autophagy detection
- DAPGreen / DAPRed (Autophagosome detection), DALGreen (Autolysosome detection)
|
- Cell cycle assay
- Cell Cycle Assay Solution Deep Red and Blue
|
- Lysosomal pH and mass detection
- Lysosomal Acidic pH Detection Kit-Green/Red and Green/Deep Red
|
- Glycolysis/Oxidative phosphorylation Assay
- Glycolysis/OXPHOS Assay Kit
|
|
|
|
|
Tracing autophagosome to autolysosome in live cells
Nampt inhibitor, FK866 inhibits the progress of autophagosome to autolysosome by lysosomal deacidification. A recent finding shows that the dysfunctional condition of nicotinamide adenine dinucleotide (NAD+) biosynthetic enzyme, Nampt induces lysosomal deacidification1). In this section, we tried to determine how NAD+ depletion-induced lysosomal deacidification affects the autophagy-lysosomal pathway. 1) Mikako Yagi, et. al., EMBO J., 40(8), e105268 (2021)
-
<Impact on Lysosomal Acidification and pH Detection>
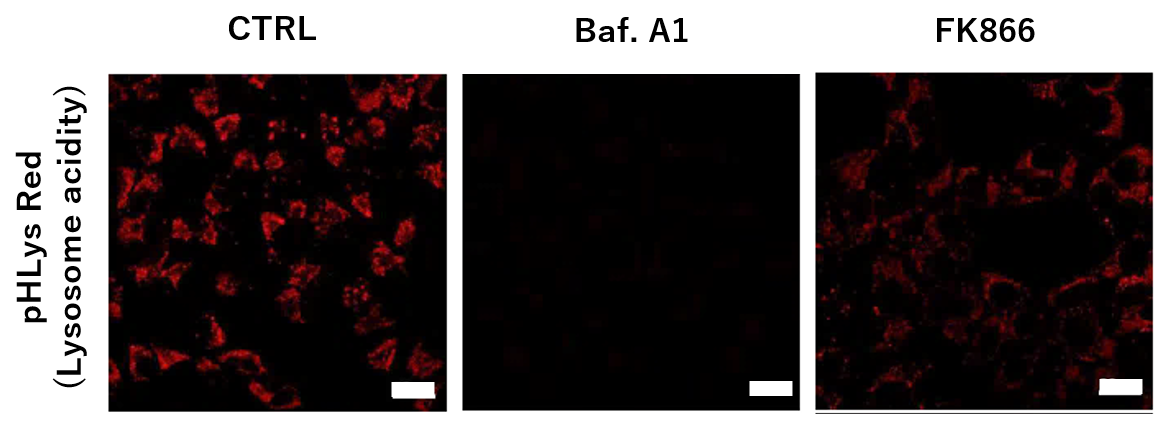
-
To confirm the effect of the Nampt inhibitor, FK866, on lysosomal acidification, HeLa cells were first labeled by the lysosomal pH detection dye pHLys Red. The cells were then treated with FK866, and lysosomal acidification inhibitor Bafilomycin A1 was used as a positive control. FK866 and Bafilomycin A1-treatment each decreased the fluorescent pHLys Red signal, indicating lysosome neutralization.
(Protocol)
HeLa cells (8 well ibidi) (MEM, FBS+)
↓
Wash x2 (HBSS)
↓
. pHLys Red working solution (HBSS, 1,000 times dilution)
. pHLys Red working solution (HBSS, 1,000 times dilution) + 50 nM Bafilomycin
A1
. pHLys Red working solution (HBSS, 1,000 times dilution) + 10 nM FK866
↓
30 min, 37°C
↓
Observed by confocal microscopy (x20)
|
-
<NAD+ Depletion and Autophagy-Lysosomal Pathway Response>
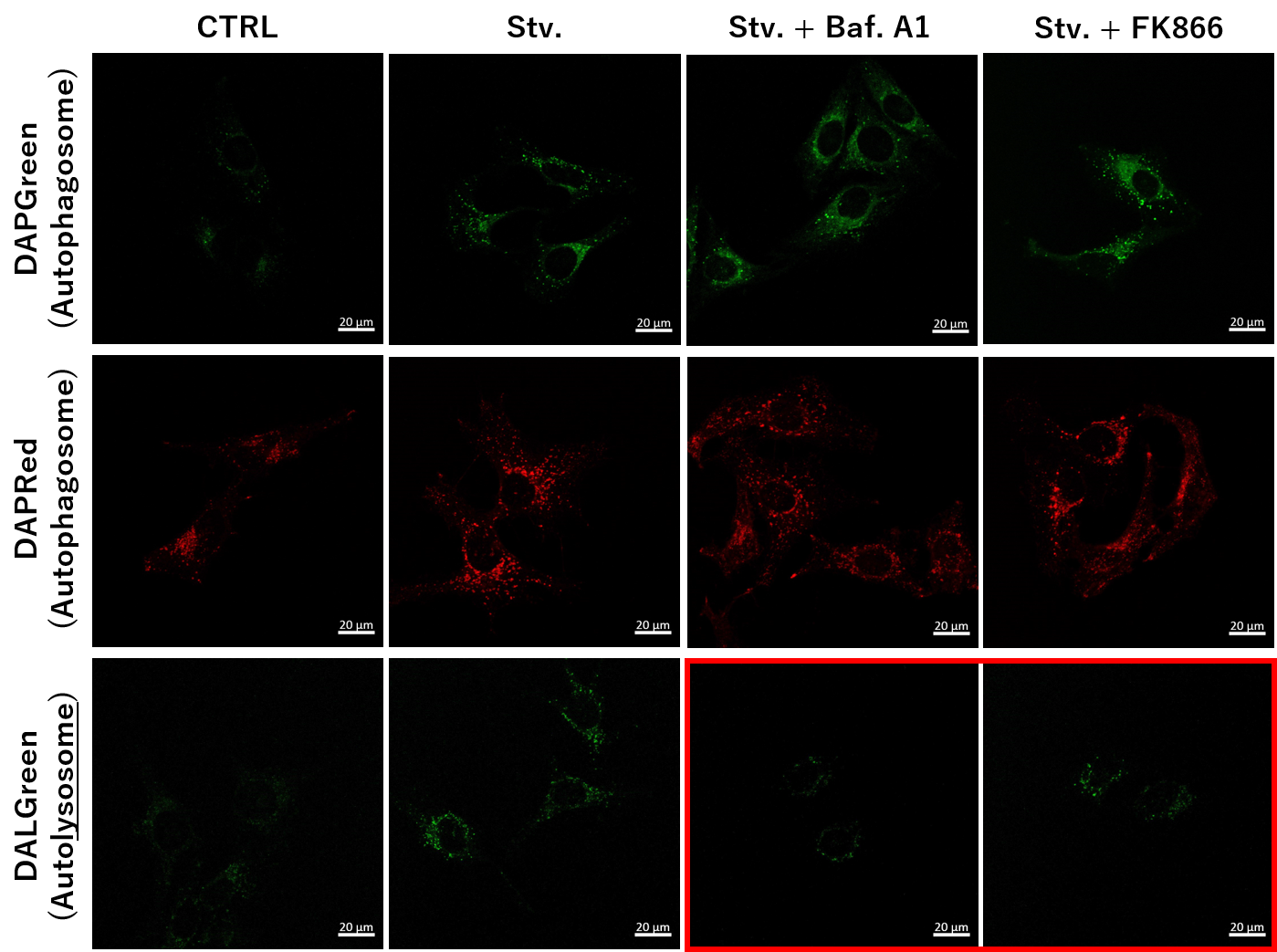
-
We next determined how FK866-induced lysosomal deacidification affects the autophagy-lysosomal pathway. After staining with DAPGreen/DAPRed (for detecting autophagosome), or DALGreen (for detecting autolysosome), HeLa cells were starved in HBSS incubation and then treated with FK866 or Bafilomycin A1. Under the starvation condition, the fluorescent signals from all dyes increased, indicating the proceeding autophagy-lysosomal pathway. On the other hand, only DALGreen's signals were decreased in FK866 and Bafilomycin A1 treated cells with starvation conditions. These results clearly suggested that FK866 inhibits the autophagy-lysosomal pathway by NAD+ depletion-induced lysosomal deacidification.
(Protocol)
HeLa cells (8 well ibidi) (MEM, FBS+)
↓
Single-stain with 2 umol/I DALGreen or
0.2 umol/I DAPGreen or
0.2 umol/I DAPRed
30 min, 37℃
↓
Wash x2 (MEM, FBS+)
↓
. Control (MEM, FBS+) for 2 h 20 min
· Starvation (HBSS) for 2 h 20 min
· Stv.2 h = 10 nM Bafilomycin A1 (HBSS) for 20 min
· Stv.2 h = 10 nM FK866 (HBSS) for 20 min
↓
Observed by confocal microscopy (x40)
|

















