Total Glutathione Quantification Kit
Glutathione Quantification
-
Product codeT419 Total Glutathione Quantification Kit
| Unit size | Price | FUJIFILM Wako Products code |
|---|---|---|
| 100 tests | ¥36,500 | 348-90201 |
| 100 tests | ・Substrate(DTNB) ・Enzyme Solution 50 μL ・Coenzyme ・Buffer Solution 50 ml ・Standard GSH |
x2 x1 x2 x1 x1 |
|---|
Description
Glutathione (GSH) is the most abundant thiol compound in animal tissues, plant tissues, bacteria, and yeast. GSH has many different roles, including protection against reactive oxygen species and the maintenance of protein thiol groups. During these processes, GSH is converted into its oxidized form, glutathione disulfide (GSSG). Since GSSG is then enzymatically reduced by glutathione reductase, GSH is the dominant form in organisms. DTNB (5,5 EDithiobis(2-nitrobenzoic acid)), known as Ellman’s Reagent, was developed to detect thiol compounds. In 1985, Dr. M. E. Anderson suggested that the glutathione recycling system involving DTNB and glutathione reductase could be used as a highly sensitive glutathione detection method. DTNB and GSH react to generate 5-Mercapto-2-nitrobenzoic acid (TNB) in Fig. 1. Since TNB is yellow, the GSH concentration in a sample solution can be determined by O.D. measurement at 412 nm absorbance (Fig. 2). GSH is regenerated from GS-TNB by glutathione reductase and will again react with DTNB to produce TNB. This recycling reaction improves the sensitivity of total glutathione detection. Total Glutathione Quantification Kit contains all of the necessary reagents for total glutathione measurement, except for those used in sample preparation. 5-Sulfosalicylic acid is recommended for the removal of proteins from sample solutions and for the prevention of GSH oxidation and γ-glutamyl transpeptidase reactions. However, the optimum method for sample preparation differs from sample to sample, so please review the references. This kit can be used to quantify total glutathione concentrations from 1 μM to 100 μM using the standard method. For lower glutathione concentrations, such as in blood samples, longer incubation times are required.
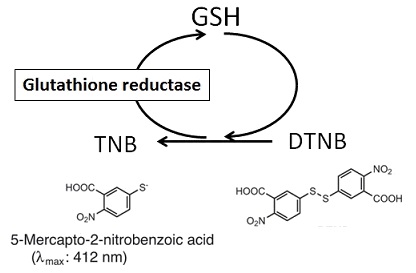
Fig. 1 Mechanism of total glutathione quantification
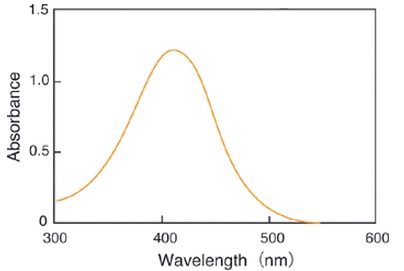
Fig. 2 Absorption spectrum of 5-Mercapto-2-nitrobenzoic acid in phosphate buffer (pH 7.5)
| Developer | Dojindo Molecular Technologies, Inc. |
|---|
Manual
Technical info
Preparation of Various Sample Solution
Cells (Adhesive cells: 5×105 cells; Leukocyte cells: 1×106 cells)
1. Collect cells by centrifugation at 200 g for 10 min at 4°C. Discard the supernatant.
2. Wash the cells with 300 μl PBS and centrifuge at 200 g for 10 min at 4°C. Discard the supernatant.
3. Add 80 μl 10 mM HCl, and lyse the cells by freezing and thawing twice.
4. Add 20 μl 5% SSA and centrifuge at 8,000 g for 10 min.
5. Transfer the supernatant to a new tube, and use it for the assay. If the final concentration of SSA is over 1%, add ddH2O to reduce the concentration of SSA from 0.5 to 1%.
Tissue (100 mg)
1. Homogenize the tissue in 0.5-1.0 ml 5% SSA.
2. Centrifuge the homogenized tissue sample at 8,000 g for 10 min.
3. Transfer the supernatant to a new tube and add ddH2O to reduce the concentration of SSA from 0.5 to 1%. Use it for the assay.
Plasma
1. Centrifuge anticoagulant-treated blood at 1,000 g for 10 min at 4°C.
2. Transfer the top plasma layer to a new tube and add 5% SSA equivalent to half of the volume of the plasma.
3. Centrifuge at 8,000 g for 10 min at 4°C
4. Transfer the supernatant to a new tube, and add ddH2O to reduce the concentration of SSA from 0.5 to 1%. Use it for the assay.
Erythrocytes
1. Centrifuge anticoagulant-treated blood at 1,000 g for 10 min at 4°C.
2. Discard the supernatant and the white buffy layer.
3. Lyse the erythrocytes with 5% SSA equivalent to 4 times the volume of the erythrocytes.
4. Centrifuge at 8,000 g for 10 min at 4°C.
5. Transfer the supernatant to a new tube, and add ddH2O to reduce the concentration of SSA from 0.5 to 1%. Use it for the assay. Erythrocytes can be isolated from the remaining sample solution after the plasma sample isolation.
Preparation of Assay Solutions
Preparation of 5% 5-Sulfosalicylic Acid (SSA) Solution
Note: SSA is not included in this kit.
1. Dissolve 1 g SSA in 19 ml water.
2. Store the solution at 4°C (stable for 6 months at 4°C).
Substrate Working Solution
Add 1 ml Buffer Solution to 1 vial of Substrate, and dissolve. Substrate working solution is stable for 2 months at -20ºC.
Enzyme Working Solution
Mix Enzyme solution with pipetting before using. Take out 20 μl Enzyme solution and mix it with 4 ml Buffer solution. Enzyme working solution is stable for 2 months at 4ºC.
Coenzyme Working Solution
Add 0.7 ml ddH2O to the Coenzyme vial and dissolve. If you don’t use all of the coenzyme working solution in one day, aliquot it into microtubes and store at -20ºC. If you use all of the coenzyme working solution in one day, just add 6.3 ml Buffer solution to the vial.
The Coenzyme vial is under vacuum pressure; carefully open the cap or use a syringe to add Buffer solution. Since the Coenzyme working solution dissolved in the Buffer solution is not stable, use it in one day. The coenzyme solution prepared with ddH2O is only stable for 2 months at -20oC. Dilute 10 times with Buffer solution to prepare Working solution prior to use.
GSH Standard Solutions
To prepare 200 μM GSH standard solution, add 2 ml of 0.5-1% SSA to the Standard GSH vial and dissolve. Dilute 100 μl of the 200 μM GSH standard solution with 100 μl of 0.5% SSA, and repeat using serial dilution to prepare the following GSH standard solutions:
100 μM, 50 μM, 25 μM, 12.5 μM, 6.25 μM, 3.13 μM, 1.56 μM and 0.
The Standard GSH vial is under vacuum pressure; carefully open the cap or use a syringe to add SSA. GSH powder is difficult to see. The GSH standard solutions are stable for 2 months at -20°C.
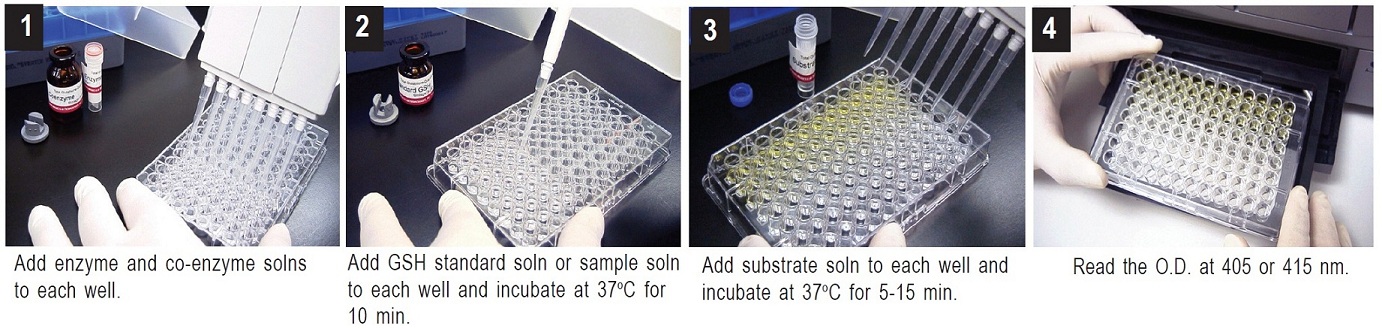
Total Glutathione Detection – Standard Method
Detection Range: 5-100 μM
1. To each well, add 20 μl of Enzyme working solution, 140 μl of Coenzyme working solution, and 20 μl of either one of the GSH standard solutions or the sample solution.a)
2. Incubate the plate at 37°C for 10 min.
3. Add 20 μl of Substrate working solution, and incubate the plate at 37°C for 5-10 min.
4. Read the absorbance at 405 nm or 415 nm using a microplate reader.
5. Determine the concentration of GSH in the sample solution using a calibration curveb).
a) Adjust the concentration of SSA in the sample solution to 0.5-1% with ddH2O before the assay. High concentrations of SSA (>1 %) interfere with the assay.
b) Since the colorimetric reaction is stable and the O.D. increases linearly over 30 min, GSH concentration can be determined by kinetic or pseudo-endpoint (no stopping reaction, quick measurement of O.D. at certain time periods between 5 and 10 min) methods.
Total Glutathione Detection – High Sensitivity Method Detection Range: 0.5-25 μM
1. To each well, add 20 μl of Enzyme working solution, 140 μl of Coenzyme working solution, and 20 μl of either one of the GSH standard solutionsa) or the sample solutionb).
2. Incubate the plate at 30°C for 10 min.
3. Add 20 μl of Substrate working solution, and incubate the plate at 37°C for 20-40 min.
4. Read the absorbance at 405 nm or 415 nm using a microplate reader.
5. Determine the concentration of GSH in the sample solution using a calibration curve.
a) Prepare 50 mM GSH standard solution, and then prepare different concentrations of GSH standard solutions by serial dilution with 0.5% SSA as follows: 25 μM, 12.5 μM, 6.25 μM, 3.13 μM, 1.56 μM, 0.78 μM, 0.39 μM and 0.
b) Adjust the concentration of SSA in the sample solution to 0.5-1% with ddH2O before the assay. Higher concentrations of SSA (>1%) interfere with the assay.
Determination of Total Glutathione (GSH and GSSG) Concentration
Determine the total glutathione concentration in the sample solution using the following equations. Since the values obtained by these equations are the amount of total glutathione in treated sample solutions, further calculations are necessary if the actual concentration of glutathione in cells or tissues needs to be determined.
Calibration Curve
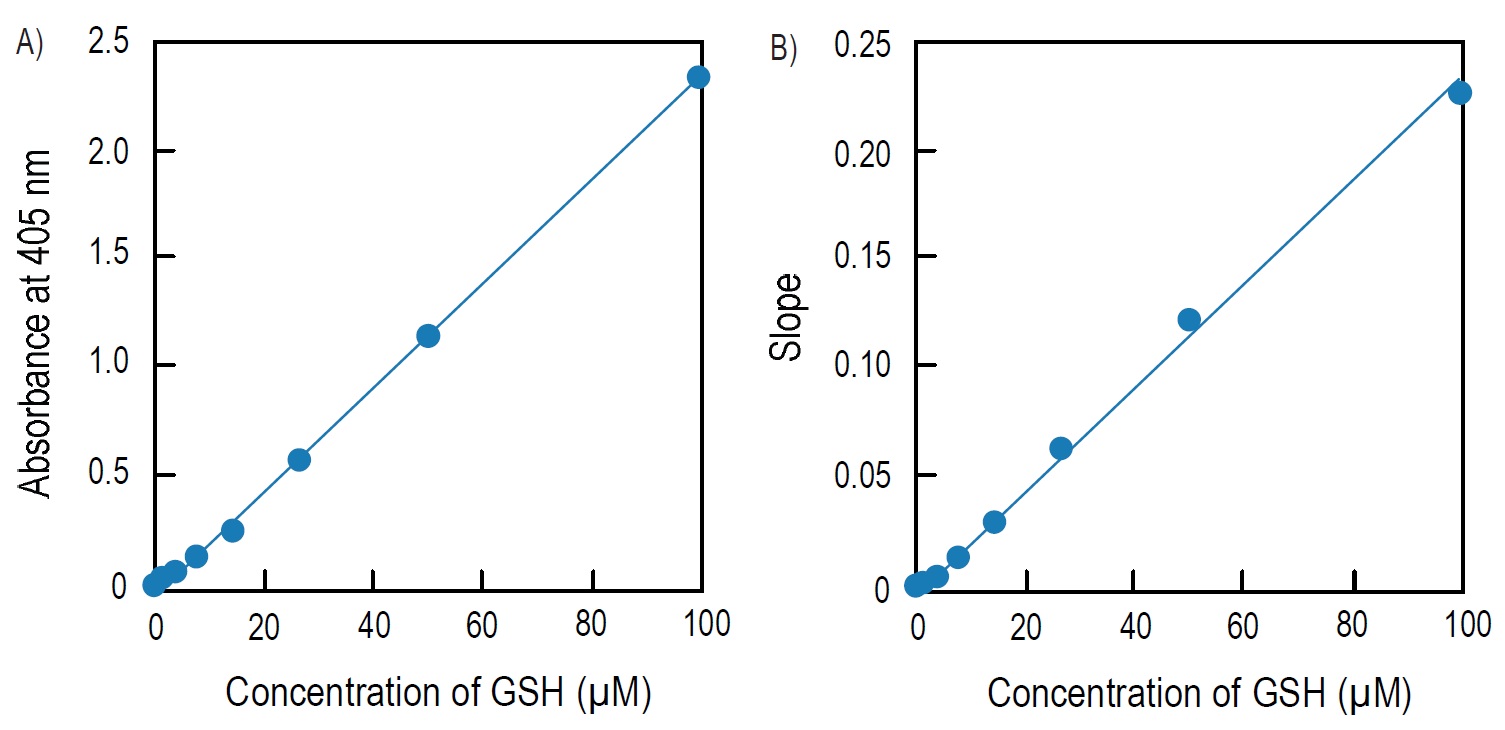
Fig. 4 Calibration curves prepared using pseudo-endpoint method and kinetic method
A) Calibration curve prepared using pseudo-endpoint method. 10 min incubation at room temperature.
B) Calibration curve prepared using kinetic method.
Pseudo-endpoint method:
Total glutathione = (O.D.sample O.D.blank) / slopea)
Kinetic method:
Total glutathione = (Slopesampleb)-Slopeblankb))/slopeb)
a)The slope of the calibration curve prepared by the pseudo-endpoint or kinetic method.
b)The slope of the kinetic reaction.
References
1) O. W. Griffith,"Determination of Glutathione and Glutathione Disulfide Using Glutathione Reductase and 2-Vinylpyridine", Anal. Biochem., 1980, 106, 207.
2) M. E. Anderson, "Determination of Glutathione and Glutathione Disulfide in Biological Samples", Methods in Enzymol., 1985, 113, 548.
3) M. A. Baker, G. J. Cerniglia and A. Zaman, "Microtiter Plate Assay for the Measurement of Glutathione and Glutathione Disulfide in Large Numbers of Biological Samples", Anal. Biochem., 1990, 190, 360.
4) C. Vandeputte, I. Guizon, I. Genestie-Denis, B. Vannier and G. Lorenzon, "A Micrototer Plate Assay for Total Glutathione and Glutathione Disulfide Contents in Cultured/isolated Cells: Performance Study of a New Miniaturized Protocol", Cell Biol. Toxicol., 1994, 10, 415.
5) S. A. McGrath-Morrow and J. Stahl,"Inhibition of Glutamine Synthetase in A549 Cells During Hyperoxia", Am. J. Respir. Cell Mol. Biol., 2002, 27, 99.
6) T. Sato, K. Seyama, Y. Sato, H. Mori, S. Souma, T. Akiyoshi, Y. Kodama, T. Mori, S. Goto, K. Takahashi, Y. Fukuchi, N. Maruyama and A. Ishigami, "Senescence Marker Protein-30 Protects Mice Lungs from Oxidative Stress, Aging, and Smoking", Am. J. Respir. Crit. Care Med., 2006, 174, 530.
7) M. L. Mulhern, C. J. Madson, A. Danford, K. Ikesugi, P. F. Kador and T. Shinohara, "The Unfolded Protein Response in Lens Epithelial Cells from Galactosemic Rat Lenses", Invest. Ophthalmol. Vis. Sci., 2006, 47(9), 3951.
Q & A
-
Q
Do I have to dilute the sample solution prior to the assay?
-
A
If you do not know the total glutathione level of your sample, multiple dilutions may be necessary. If the total glutathione level of your sample is less than 100 μM, no dilution is necessary.
-
Q
What interferes with the assay?
-
A
Reducing agents (such as ascorbic acid, beta-mercaptoethanol, dithiothreitol, and cysteine) and thiol reactive compounds (such as maleimides) interfere with the glutathione assay. Therefore, reducing agents and thiol reactive compounds should be avoided during the sample preparation.
Handling and storage condition
| 0-5°C |